Narges Razavian
3D Foundation AI Model for Generalizable Disease Detection in Head Computed Tomography
Feb 04, 2025



Abstract:Head computed tomography (CT) imaging is a widely-used imaging modality with multitudes of medical indications, particularly in assessing pathology of the brain, skull, and cerebrovascular system. It is commonly the first-line imaging in neurologic emergencies given its rapidity of image acquisition, safety, cost, and ubiquity. Deep learning models may facilitate detection of a wide range of diseases. However, the scarcity of high-quality labels and annotations, particularly among less common conditions, significantly hinders the development of powerful models. To address this challenge, we introduce FM-CT: a Foundation Model for Head CT for generalizable disease detection, trained using self-supervised learning. Our approach pre-trains a deep learning model on a large, diverse dataset of 361,663 non-contrast 3D head CT scans without the need for manual annotations, enabling the model to learn robust, generalizable features. To investigate the potential of self-supervised learning in head CT, we employed both discrimination with self-distillation and masked image modeling, and we construct our model in 3D rather than at the slice level (2D) to exploit the structure of head CT scans more comprehensively and efficiently. The model's downstream classification performance is evaluated using internal and three external datasets, encompassing both in-distribution (ID) and out-of-distribution (OOD) data. Our results demonstrate that the self-supervised foundation model significantly improves performance on downstream diagnostic tasks compared to models trained from scratch and previous 3D CT foundation models on scarce annotated datasets. This work highlights the effectiveness of self-supervised learning in medical imaging and sets a new benchmark for head CT image analysis in 3D, enabling broader use of artificial intelligence for head CT-based diagnosis.
Automatic Detection of Alzheimer's Disease with Multi-Modal Fusion of Clinical MRI Scans
Nov 30, 2023Abstract:The aging population of the U.S. drives the prevalence of Alzheimer's disease. Brookmeyer et al. forecasts approximately 15 million Americans will have either clinical AD or mild cognitive impairment by 2060. In response to this urgent call, methods for early detection of Alzheimer's disease have been developed for prevention and pre-treatment. Notably, literature on the application of deep learning in the automatic detection of the disease has been proliferating. This study builds upon previous literature and maintains a focus on leveraging multi-modal information to enhance automatic detection. We aim to predict the stage of the disease - Cognitively Normal (CN), Mildly Cognitive Impairment (MCI), and Alzheimer's Disease (AD), based on two different types of brain MRI scans. We design an AlexNet-based deep learning model that learns the synergy of complementary information from both T1 and FLAIR MRI scans.
Making Self-supervised Learning Robust to Spurious Correlation via Learning-speed Aware Sampling
Nov 29, 2023Abstract:Self-supervised learning (SSL) has emerged as a powerful technique for learning rich representations from unlabeled data. The data representations are able to capture many underlying attributes of data, and be useful in downstream prediction tasks. In real-world settings, spurious correlations between some attributes (e.g. race, gender and age) and labels for downstream tasks often exist, e.g. cancer is usually more prevalent among elderly patients. In this paper, we investigate SSL in the presence of spurious correlations and show that the SSL training loss can be minimized by capturing only a subset of the conspicuous features relevant to those sensitive attributes, despite the presence of other important predictive features for the downstream tasks. To address this issue, we investigate the learning dynamics of SSL and observe that the learning is slower for samples that conflict with such correlations (e.g. elder patients without cancer). Motivated by these findings, we propose a learning-speed aware SSL (LA-SSL) approach, in which we sample each training data with a probability that is inversely related to its learning speed. We evaluate LA-SSL on three datasets that exhibit spurious correlations between different attributes, demonstrating that it improves the robustness of pretrained representations on downstream classification tasks.
Multiple Instance Learning via Iterative Self-Paced Supervised Contrastive Learning
Oct 17, 2022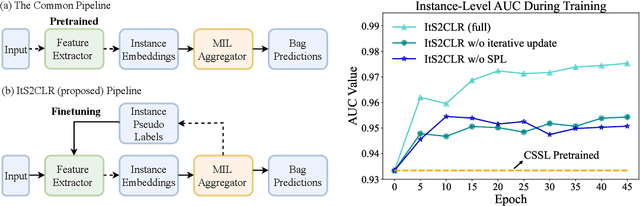
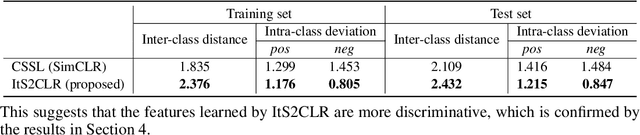
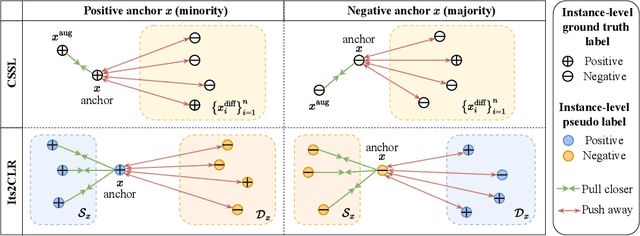

Abstract:Learning representations for individual instances when only bag-level labels are available is a fundamental challenge in multiple instance learning (MIL). Recent works have shown promising results using contrastive self-supervised learning (CSSL), which learns to push apart representations corresponding to two different randomly-selected instances. Unfortunately, in real-world applications such as medical image classification, there is often class imbalance, so randomly-selected instances mostly belong to the same majority class, which precludes CSSL from learning inter-class differences. To address this issue, we propose a novel framework, Iterative Self-paced Supervised Contrastive Learning for MIL Representations (ItS2CLR), which improves the learned representation by exploiting instance-level pseudo labels derived from the bag-level labels. The framework employs a novel self-paced sampling strategy to ensure the accuracy of pseudo labels. We evaluate ItS2CLR on three medical datasets, showing that it improves the quality of instance-level pseudo labels and representations, and outperforms existing MIL methods in terms of both bag and instance level accuracy.
Interpretable Prediction of Lung Squamous Cell Carcinoma Recurrence With Self-supervised Learning
Mar 23, 2022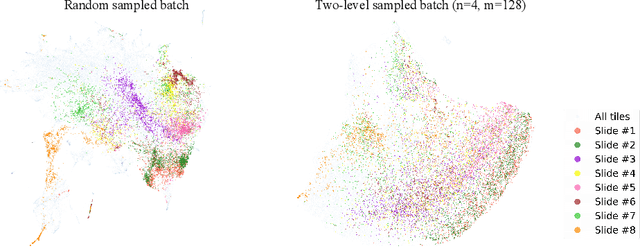
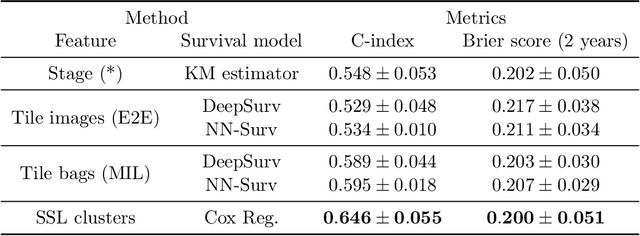
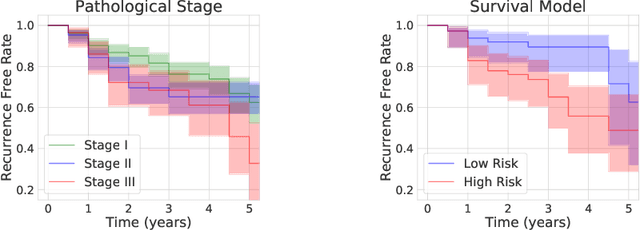

Abstract:Lung squamous cell carcinoma (LSCC) has a high recurrence and metastasis rate. Factors influencing recurrence and metastasis are currently unknown and there are no distinct histopathological or morphological features indicating the risks of recurrence and metastasis in LSCC. Our study focuses on the recurrence prediction of LSCC based on H&E-stained histopathological whole-slide images (WSI). Due to the small size of LSCC cohorts in terms of patients with available recurrence information, standard end-to-end learning with various convolutional neural networks for this task tends to overfit. Also, the predictions made by these models are hard to interpret. Histopathology WSIs are typically very large and are therefore processed as a set of smaller tiles. In this work, we propose a novel conditional self-supervised learning (SSL) method to learn representations of WSI at the tile level first, and leverage clustering algorithms to identify the tiles with similar histopathological representations. The resulting representations and clusters from self-supervision are used as features of a survival model for recurrence prediction at the patient level. Using two publicly available datasets from TCGA and CPTAC, we show that our LSCC recurrence prediction survival model outperforms both LSCC pathological stage-based approach and machine learning baselines such as multiple instance learning. The proposed method also enables us to explain the recurrence histopathological risk factors via the derived clusters. This can help pathologists derive new hypotheses regarding morphological features associated with LSCC recurrence.
Deep Probability Estimation
Nov 21, 2021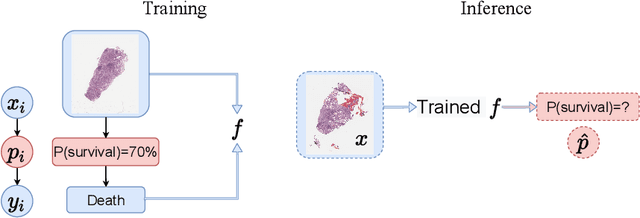
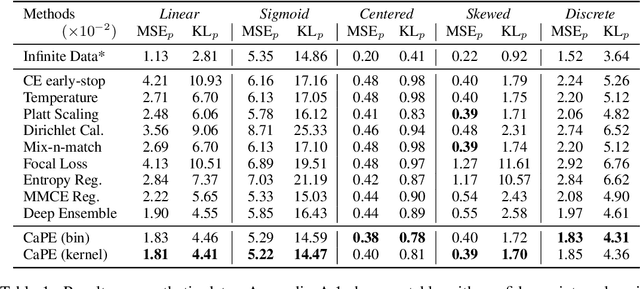
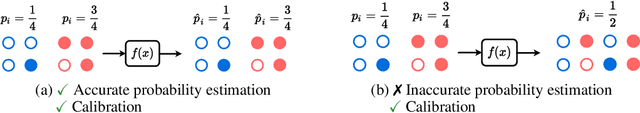
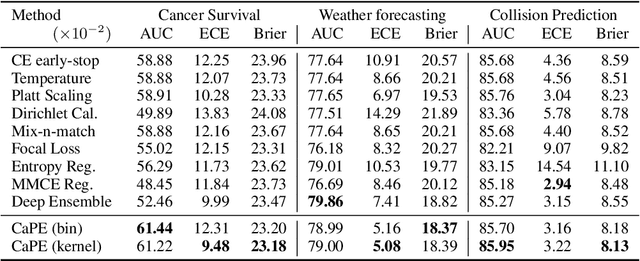
Abstract:Reliable probability estimation is of crucial importance in many real-world applications where there is inherent uncertainty, such as weather forecasting, medical prognosis, or collision avoidance in autonomous vehicles. Probability-estimation models are trained on observed outcomes (e.g. whether it has rained or not, or whether a patient has died or not), because the ground-truth probabilities of the events of interest are typically unknown. The problem is therefore analogous to binary classification, with the important difference that the objective is to estimate probabilities rather than predicting the specific outcome. The goal of this work is to investigate probability estimation from high-dimensional data using deep neural networks. There exist several methods to improve the probabilities generated by these models but they mostly focus on classification problems where the probabilities are related to model uncertainty. In the case of problems with inherent uncertainty, it is challenging to evaluate performance without access to ground-truth probabilities. To address this, we build a synthetic dataset to study and compare different computable metrics. We evaluate existing methods on the synthetic data as well as on three real-world probability estimation tasks, all of which involve inherent uncertainty: precipitation forecasting from radar images, predicting cancer patient survival from histopathology images, and predicting car crashes from dashcam videos. Finally, we also propose a new method for probability estimation using neural networks, which modifies the training process to promote output probabilities that are consistent with empirical probabilities computed from the data. The method outperforms existing approaches on most metrics on the simulated as well as real-world data.
Causal Effect Variational Autoencoder with Uniform Treatment
Nov 16, 2021
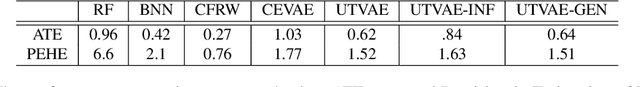

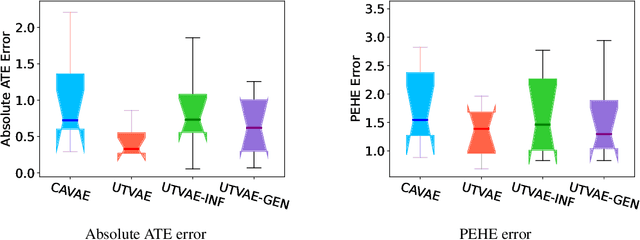
Abstract:Causal effect variational autoencoder (CEVAE) are trained to predict the outcome given observational treatment data, while uniform treatment variational autoencoders (UTVAE) are trained with uniform treatment distribution using importance sampling. In this paper, we show that using uniform treatment over observational treatment distribution leads to better causal inference by mitigating the distribution shift that occurs from training to test time. We also explore the combination of uniform and observational treatment distributions with inference and generative network training objectives to find a better training procedure for inferring treatment effect. Experimentally, we find that the proposed UTVAE yields better absolute average treatment effect error and precision in estimation of heterogeneous effect error than the CEVAE on synthetic and IHDP datasets.
Intermediate Layers Matter in Momentum Contrastive Self Supervised Learning
Oct 27, 2021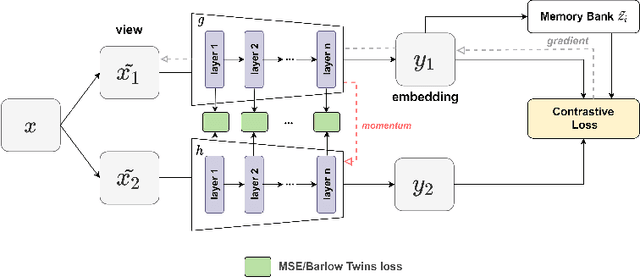
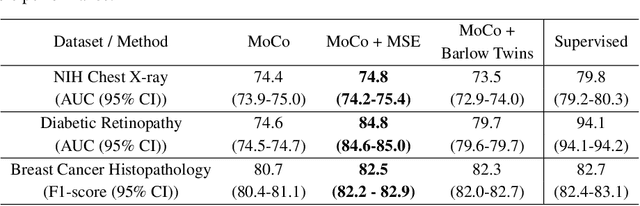
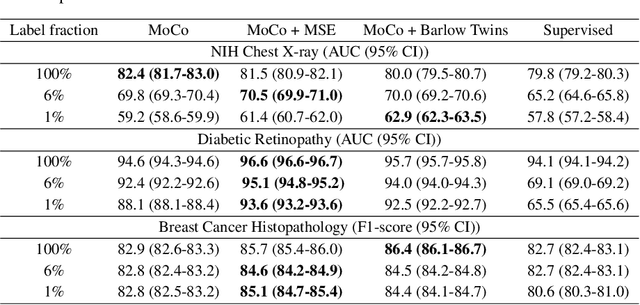
Abstract:We show that bringing intermediate layers' representations of two augmented versions of an image closer together in self-supervised learning helps to improve the momentum contrastive (MoCo) method. To this end, in addition to the contrastive loss, we minimize the mean squared error between the intermediate layer representations or make their cross-correlation matrix closer to an identity matrix. Both loss objectives either outperform standard MoCo, or achieve similar performances on three diverse medical imaging datasets: NIH-Chest Xrays, Breast Cancer Histopathology, and Diabetic Retinopathy. The gains of the improved MoCo are especially large in a low-labeled data regime (e.g. 1% labeled data) with an average gain of 5% across three datasets. We analyze the models trained using our novel approach via feature similarity analysis and layer-wise probing. Our analysis reveals that models trained via our approach have higher feature reuse compared to a standard MoCo and learn informative features earlier in the network. Finally, by comparing the output probability distribution of models fine-tuned on small versus large labeled data, we conclude that our proposed method of pre-training leads to lower Kolmogorov-Smirnov distance, as compared to a standard MoCo. This provides additional evidence that our proposed method learns more informative features in the pre-training phase which could be leveraged in a low-labeled data regime.
An artificial intelligence system for predicting the deterioration of COVID-19 patients in the emergency department
Aug 04, 2020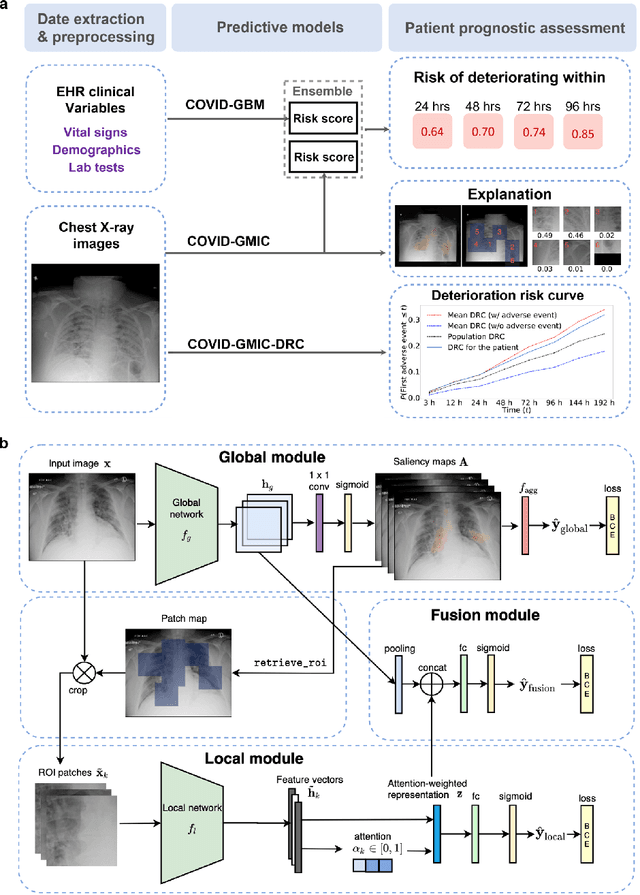
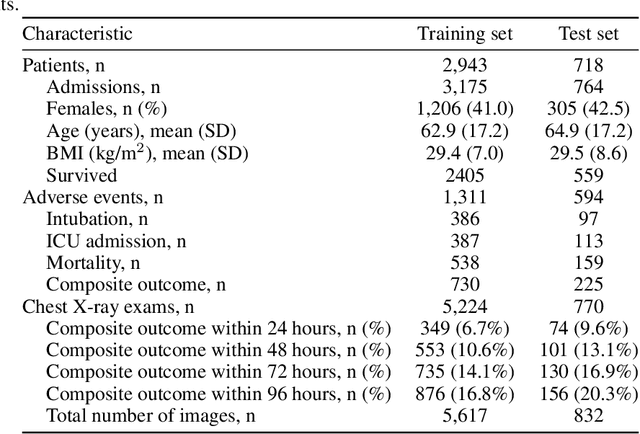
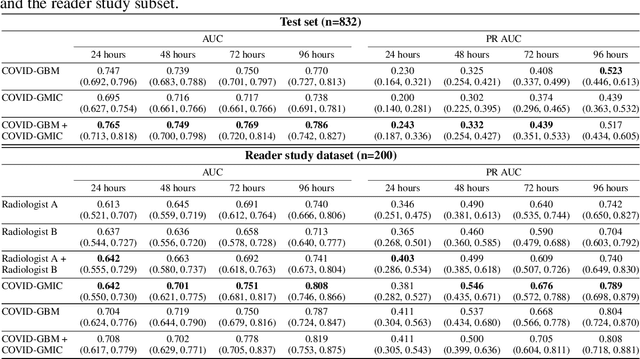
Abstract:During the COVID-19 pandemic, rapid and accurate triage of patients at the emergency department is critical to inform decision-making. We propose a data-driven approach for automatic prediction of deterioration risk using a deep neural network that learns from chest X-ray images, and a gradient boosting model that learns from routine clinical variables. Our AI prognosis system, trained using data from 3,661 patients, achieves an AUC of 0.786 (95% CI: 0.742-0.827) when predicting deterioration within 96 hours. The deep neural network extracts informative areas of chest X-ray images to assist clinicians in interpreting the predictions, and performs comparably to two radiologists in a reader study. In order to verify performance in a real clinical setting, we silently deployed a preliminary version of the deep neural network at NYU Langone Health during the first wave of the pandemic, which produced accurate predictions in real-time. In summary, our findings demonstrate the potential of the proposed system for assisting front-line physicians in the triage of COVID-19 patients.
Early-Learning Regularization Prevents Memorization of Noisy Labels
Jun 30, 2020



Abstract:We propose a novel framework to perform classification via deep learning in the presence of noisy annotations. When trained on noisy labels, deep neural networks have been observed to first fit the training data with clean labels during an "early learning" phase, before eventually memorizing the examples with false labels. We prove that early learning and memorization are fundamental phenomena in high-dimensional classification tasks, even in simple linear models, and give a theoretical explanation in this setting. Motivated by these findings, we develop a new technique for noisy classification tasks, which exploits the progress of the early learning phase. In contrast with existing approaches, which use the model output during early learning to detect the examples with clean labels, and either ignore or attempt to correct the false labels, we take a different route and instead capitalize on early learning via regularization. There are two key elements to our approach. First, we leverage semi-supervised learning techniques to produce target probabilities based on the model outputs. Second, we design a regularization term that steers the model towards these targets, implicitly preventing memorization of the false labels. The resulting framework is shown to provide robustness to noisy annotations on several standard benchmarks and real-world datasets, where it achieves results comparable to the state of the art.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge