Luyi Han
Diagnostic Performance of Universal-Learning Ultrasound AI Across Multiple Organs and Tasks: the UUSIC25 Challenge
Dec 19, 2025Abstract:IMPORTANCE: Current ultrasound AI remains fragmented into single-task tools, limiting clinical utility compared to versatile modern ultrasound systems. OBJECTIVE: To evaluate the diagnostic accuracy and efficiency of single general-purpose deep learning models for multi-organ classification and segmentation. DESIGN: The Universal UltraSound Image Challenge 2025 (UUSIC25) involved developing algorithms on 11,644 images (public/private). Evaluation used an independent, multi-center test set of 2,479 images, including data from a center completely unseen during training to assess generalization. OUTCOMES: Diagnostic performance (Dice Similarity Coefficient [DSC]; Area Under the Receiver Operating Characteristic Curve [AUC]) and computational efficiency (inference time, GPU memory). RESULTS: Of 15 valid algorithms, the top model (SMART) achieved a macro-averaged DSC of 0.854 across 5 segmentation tasks and AUC of 0.766 for binary classification. Models showed high capability in segmentation (e.g., fetal head DSC: 0.942) but variability in complex tasks subject to domain shift. Notably, in breast cancer molecular subtyping, the top model's performance dropped from AUC 0.571 (internal) to 0.508 (unseen external center), highlighting generalization challenges. CONCLUSIONS: General-purpose AI models achieve high accuracy and efficiency across multiple tasks using a single architecture. However, performance degradation on unseen data suggests domain generalization is critical for future clinical deployment.
crossMoDA Challenge: Evolution of Cross-Modality Domain Adaptation Techniques for Vestibular Schwannoma and Cochlea Segmentation from 2021 to 2023
Jun 13, 2025Abstract:The cross-Modality Domain Adaptation (crossMoDA) challenge series, initiated in 2021 in conjunction with the International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI), focuses on unsupervised cross-modality segmentation, learning from contrast-enhanced T1 (ceT1) and transferring to T2 MRI. The task is an extreme example of domain shift chosen to serve as a meaningful and illustrative benchmark. From a clinical application perspective, it aims to automate Vestibular Schwannoma (VS) and cochlea segmentation on T2 scans for more cost-effective VS management. Over time, the challenge objectives have evolved to enhance its clinical relevance. The challenge evolved from using single-institutional data and basic segmentation in 2021 to incorporating multi-institutional data and Koos grading in 2022, and by 2023, it included heterogeneous routine data and sub-segmentation of intra- and extra-meatal tumour components. In this work, we report the findings of the 2022 and 2023 editions and perform a retrospective analysis of the challenge progression over the years. The observations from the successive challenge contributions indicate that the number of outliers decreases with an expanding dataset. This is notable since the diversity of scanning protocols of the datasets concurrently increased. The winning approach of the 2023 edition reduced the number of outliers on the 2021 and 2022 testing data, demonstrating how increased data heterogeneity can enhance segmentation performance even on homogeneous data. However, the cochlea Dice score declined in 2023, likely due to the added complexity from tumour sub-annotations affecting overall segmentation performance. While progress is still needed for clinically acceptable VS segmentation, the plateauing performance suggests that a more challenging cross-modal task may better serve future benchmarking.
Ordinal Learning: Longitudinal Attention Alignment Model for Predicting Time to Future Breast Cancer Events from Mammograms
Sep 10, 2024Abstract:Precision breast cancer (BC) risk assessment is crucial for developing individualized screening and prevention. Despite the promising potential of recent mammogram (MG) based deep learning models in predicting BC risk, they mostly overlook the 'time-to-future-event' ordering among patients and exhibit limited explorations into how they track history changes in breast tissue, thereby limiting their clinical application. In this work, we propose a novel method, named OA-BreaCR, to precisely model the ordinal relationship of the time to and between BC events while incorporating longitudinal breast tissue changes in a more explainable manner. We validate our method on public EMBED and inhouse datasets, comparing with existing BC risk prediction and time prediction methods. Our ordinal learning method OA-BreaCR outperforms existing methods in both BC risk and time-to-future-event prediction tasks. Additionally, ordinal heatmap visualizations show the model's attention over time. Our findings underscore the importance of interpretable and precise risk assessment for enhancing BC screening and prevention efforts. The code will be accessible to the public.
Non-Adversarial Learning: Vector-Quantized Common Latent Space for Multi-Sequence MRI
Jul 03, 2024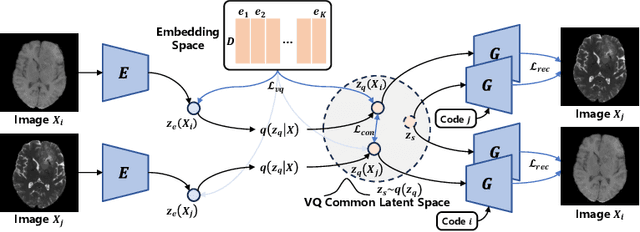
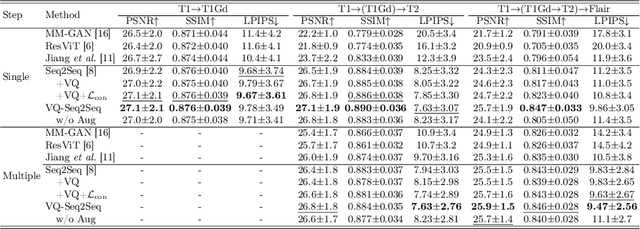
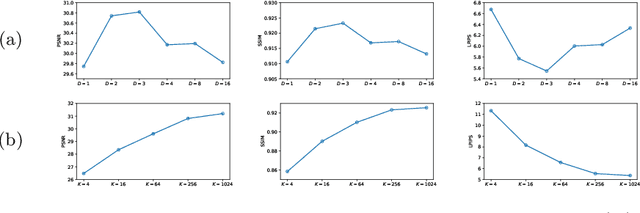

Abstract:Adversarial learning helps generative models translate MRI from source to target sequence when lacking paired samples. However, implementing MRI synthesis with adversarial learning in clinical settings is challenging due to training instability and mode collapse. To address this issue, we leverage intermediate sequences to estimate the common latent space among multi-sequence MRI, enabling the reconstruction of distinct sequences from the common latent space. We propose a generative model that compresses discrete representations of each sequence to estimate the Gaussian distribution of vector-quantized common (VQC) latent space between multiple sequences. Moreover, we improve the latent space consistency with contrastive learning and increase model stability by domain augmentation. Experiments using BraTS2021 dataset show that our non-adversarial model outperforms other GAN-based methods, and VQC latent space aids our model to achieve (1) anti-interference ability, which can eliminate the effects of noise, bias fields, and artifacts, and (2) solid semantic representation ability, with the potential of one-shot segmentation. Our code is publicly available.
To deform or not: treatment-aware longitudinal registration for breast DCE-MRI during neoadjuvant chemotherapy via unsupervised keypoints detection
Jan 17, 2024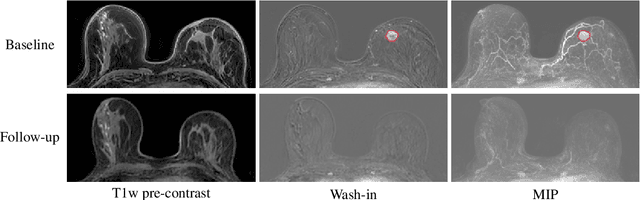
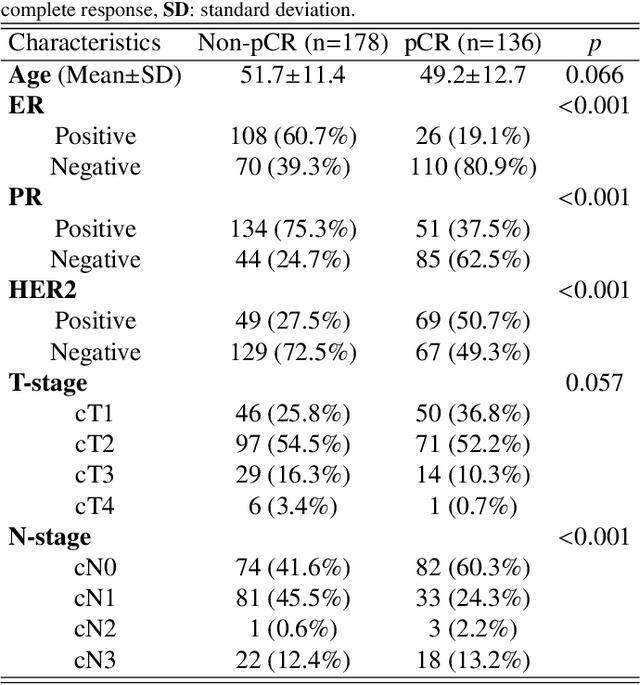
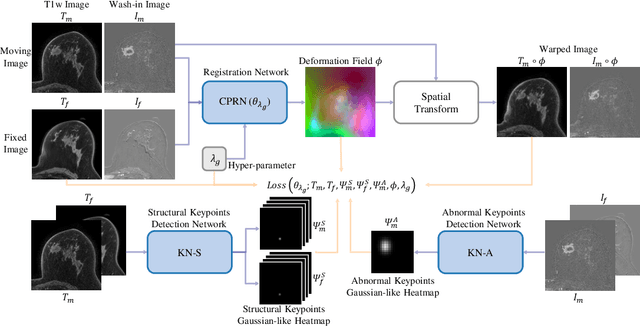
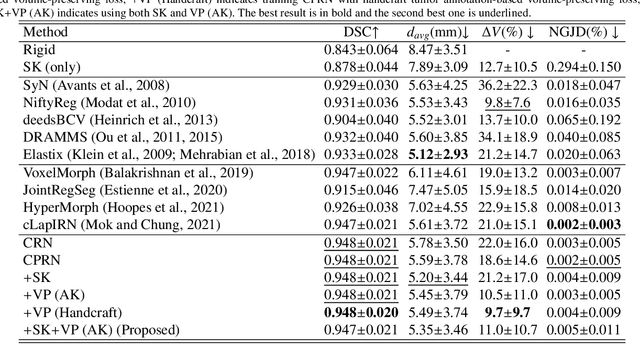
Abstract:Clinicians compare breast DCE-MRI after neoadjuvant chemotherapy (NAC) with pre-treatment scans to evaluate the response to NAC. Clinical evidence supports that accurate longitudinal deformable registration without deforming treated tumor regions is key to quantifying tumor changes. We propose a conditional pyramid registration network based on unsupervised keypoint detection and selective volume-preserving to quantify changes over time. In this approach, we extract the structural and the abnormal keypoints from DCE-MRI, apply the structural keypoints for the registration algorithm to restrict large deformation, and employ volume-preserving loss based on abnormal keypoints to keep the volume of the tumor unchanged after registration. We use a clinical dataset with 1630 MRI scans from 314 patients treated with NAC. The results demonstrate that our method registers with better performance and better volume preservation of the tumors. Furthermore, a local-global-combining biomarker based on the proposed method achieves high accuracy in pathological complete response (pCR) prediction, indicating that predictive information exists outside tumor regions. The biomarkers could potentially be used to avoid unnecessary surgeries for certain patients. It may be valuable for clinicians and/or computer systems to conduct follow-up tumor segmentation and response prediction on images registered by our method. Our code is available on \url{https://github.com/fiy2W/Treatment-aware-Longitudinal-Registration}.
Fine-Grained Unsupervised Cross-Modality Domain Adaptation for Vestibular Schwannoma Segmentation
Nov 25, 2023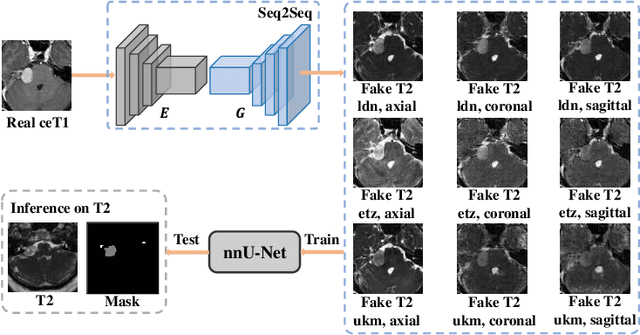
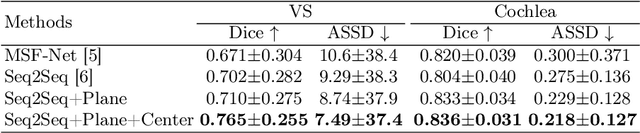
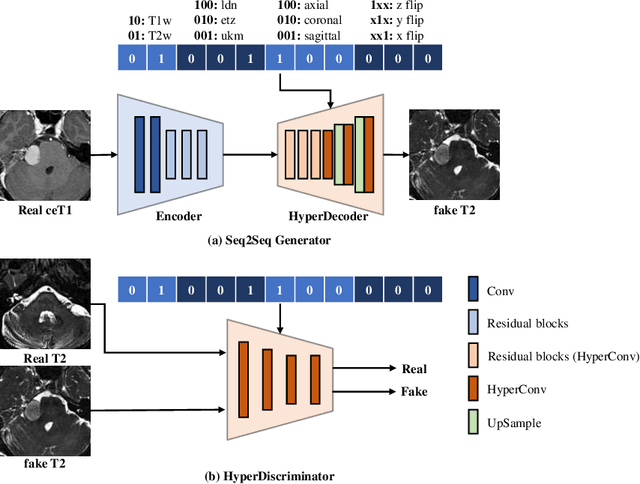
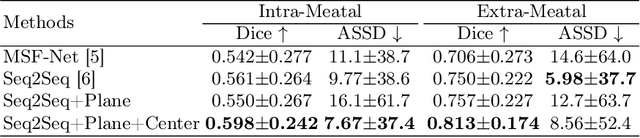
Abstract:The domain adaptation approach has gained significant acceptance in transferring styles across various vendors and centers, along with filling the gaps in modalities. However, multi-center application faces the challenge of the difficulty of domain adaptation due to their intra-domain differences. We focus on introducing a fine-grained unsupervised framework for domain adaptation to facilitate cross-modality segmentation of vestibular schwannoma (VS) and cochlea. We propose to use a vector to control the generator to synthesize a fake image with given features. And then, we can apply various augmentations to the dataset by searching the feature dictionary. The diversity augmentation can increase the performance and robustness of the segmentation model. On the CrossMoDA validation phase Leaderboard, our method received a mean Dice score of 0.765 and 0.836 on VS and cochlea, respectively.
DisAsymNet: Disentanglement of Asymmetrical Abnormality on Bilateral Mammograms using Self-adversarial Learning
Jul 06, 2023



Abstract:Asymmetry is a crucial characteristic of bilateral mammograms (Bi-MG) when abnormalities are developing. It is widely utilized by radiologists for diagnosis. The question of 'what the symmetrical Bi-MG would look like when the asymmetrical abnormalities have been removed ?' has not yet received strong attention in the development of algorithms on mammograms. Addressing this question could provide valuable insights into mammographic anatomy and aid in diagnostic interpretation. Hence, we propose a novel framework, DisAsymNet, which utilizes asymmetrical abnormality transformer guided self-adversarial learning for disentangling abnormalities and symmetric Bi-MG. At the same time, our proposed method is partially guided by randomly synthesized abnormalities. We conduct experiments on three public and one in-house dataset, and demonstrate that our method outperforms existing methods in abnormality classification, segmentation, and localization tasks. Additionally, reconstructed normal mammograms can provide insights toward better interpretable visual cues for clinical diagnosis. The code will be accessible to the public.
An Explainable Deep Framework: Towards Task-Specific Fusion for Multi-to-One MRI Synthesis
Jul 03, 2023Abstract:Multi-sequence MRI is valuable in clinical settings for reliable diagnosis and treatment prognosis, but some sequences may be unusable or missing for various reasons. To address this issue, MRI synthesis is a potential solution. Recent deep learning-based methods have achieved good performance in combining multiple available sequences for missing sequence synthesis. Despite their success, these methods lack the ability to quantify the contributions of different input sequences and estimate the quality of generated images, making it hard to be practical. Hence, we propose an explainable task-specific synthesis network, which adapts weights automatically for specific sequence generation tasks and provides interpretability and reliability from two sides: (1) visualize the contribution of each input sequence in the fusion stage by a trainable task-specific weighted average module; (2) highlight the area the network tried to refine during synthesizing by a task-specific attention module. We conduct experiments on the BraTS2021 dataset of 1251 subjects, and results on arbitrary sequence synthesis indicate that the proposed method achieves better performance than the state-of-the-art methods. Our code is available at \url{https://github.com/fiy2W/mri_seq2seq}.
Synthesis of Contrast-Enhanced Breast MRI Using Multi-b-Value DWI-based Hierarchical Fusion Network with Attention Mechanism
Jul 03, 2023



Abstract:Magnetic resonance imaging (MRI) is the most sensitive technique for breast cancer detection among current clinical imaging modalities. Contrast-enhanced MRI (CE-MRI) provides superior differentiation between tumors and invaded healthy tissue, and has become an indispensable technique in the detection and evaluation of cancer. However, the use of gadolinium-based contrast agents (GBCA) to obtain CE-MRI may be associated with nephrogenic systemic fibrosis and may lead to bioaccumulation in the brain, posing a potential risk to human health. Moreover, and likely more important, the use of gadolinium-based contrast agents requires the cannulation of a vein, and the injection of the contrast media which is cumbersome and places a burden on the patient. To reduce the use of contrast agents, diffusion-weighted imaging (DWI) is emerging as a key imaging technique, although currently usually complementing breast CE-MRI. In this study, we develop a multi-sequence fusion network to synthesize CE-MRI based on T1-weighted MRI and DWIs. DWIs with different b-values are fused to efficiently utilize the difference features of DWIs. Rather than proposing a pure data-driven approach, we invent a multi-sequence attention module to obtain refined feature maps, and leverage hierarchical representation information fused at different scales while utilizing the contributions from different sequences from a model-driven approach by introducing the weighted difference module. The results show that the multi-b-value DWI-based fusion model can potentially be used to synthesize CE-MRI, thus theoretically reducing or avoiding the use of GBCA, thereby minimizing the burden to patients. Our code is available at \url{https://github.com/Netherlands-Cancer-Institute/CE-MRI}.
GSMorph: Gradient Surgery for cine-MRI Cardiac Deformable Registration
Jun 26, 2023Abstract:Deep learning-based deformable registration methods have been widely investigated in diverse medical applications. Learning-based deformable registration relies on weighted objective functions trading off registration accuracy and smoothness of the deformation field. Therefore, they inevitably require tuning the hyperparameter for optimal registration performance. Tuning the hyperparameters is highly computationally expensive and introduces undesired dependencies on domain knowledge. In this study, we construct a registration model based on the gradient surgery mechanism, named GSMorph, to achieve a hyperparameter-free balance on multiple losses. In GSMorph, we reformulate the optimization procedure by projecting the gradient of similarity loss orthogonally to the plane associated with the smoothness constraint, rather than additionally introducing a hyperparameter to balance these two competing terms. Furthermore, our method is model-agnostic and can be merged into any deep registration network without introducing extra parameters or slowing down inference. In this study, We compared our method with state-of-the-art (SOTA) deformable registration approaches over two publicly available cardiac MRI datasets. GSMorph proves superior to five SOTA learning-based registration models and two conventional registration techniques, SyN and Demons, on both registration accuracy and smoothness.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge