Lennart Husvogt
Friedrich-Alexander-Universität Erlangen-Nürnberg Germany, Massachusetts Institute of Technology USA
A Spatiotemporal Model for Precise and Efficient Fully-automatic 3D Motion Correction in OCT
Sep 15, 2022Abstract:Optical coherence tomography (OCT) is a micrometer-scale, volumetric imaging modality that has become a clinical standard in ophthalmology. OCT instruments image by raster-scanning a focused light spot across the retina, acquiring sequential cross-sectional images to generate volumetric data. Patient eye motion during the acquisition poses unique challenges: Non-rigid, discontinuous distortions can occur, leading to gaps in data and distorted topographic measurements. We present a new distortion model and a corresponding fully-automatic, reference-free optimization strategy for computational motion correction in orthogonally raster-scanned, retinal OCT volumes. Using a novel, domain-specific spatiotemporal parametrization of forward-warping displacements, eye motion can be corrected continuously for the first time. Parameter estimation with temporal regularization improves robustness and accuracy over previous spatial approaches. We correct each A-scan individually in 3D in a single mapping, including repeated acquisitions used in OCT angiography protocols. Specialized 3D forward image warping reduces median runtime to < 9 s, fast enough for clinical use. We present a quantitative evaluation on 18 subjects with ocular pathology and demonstrate accurate correction during microsaccades. Transverse correction is limited only by ocular tremor, whereas submicron repeatability is achieved axially (0.51 um median of medians), representing a dramatic improvement over previous work. This allows assessing longitudinal changes in focal retinal pathologies as a marker of disease progression or treatment response, and promises to enable multiple new capabilities such as supersampled/super-resolution volume reconstruction and analysis of pathological eye motion occuring in neurological diseases.
Maximum a posteriori signal recovery for optical coherence tomography angiography image generation and denoising
Oct 29, 2020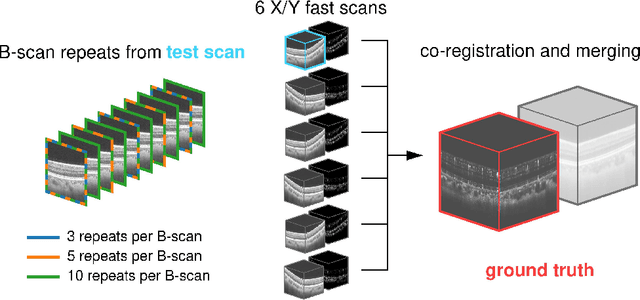
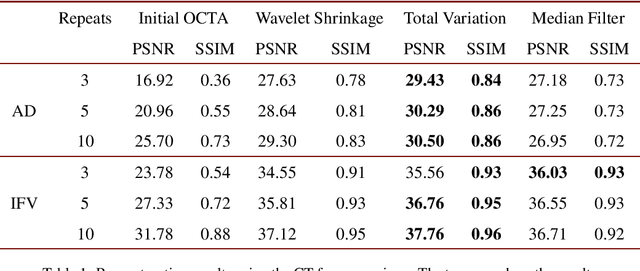
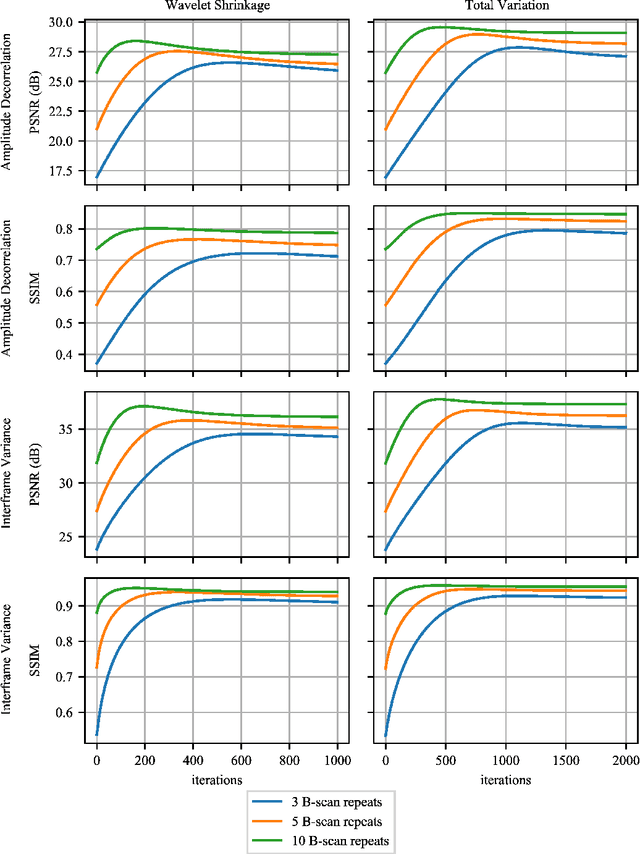
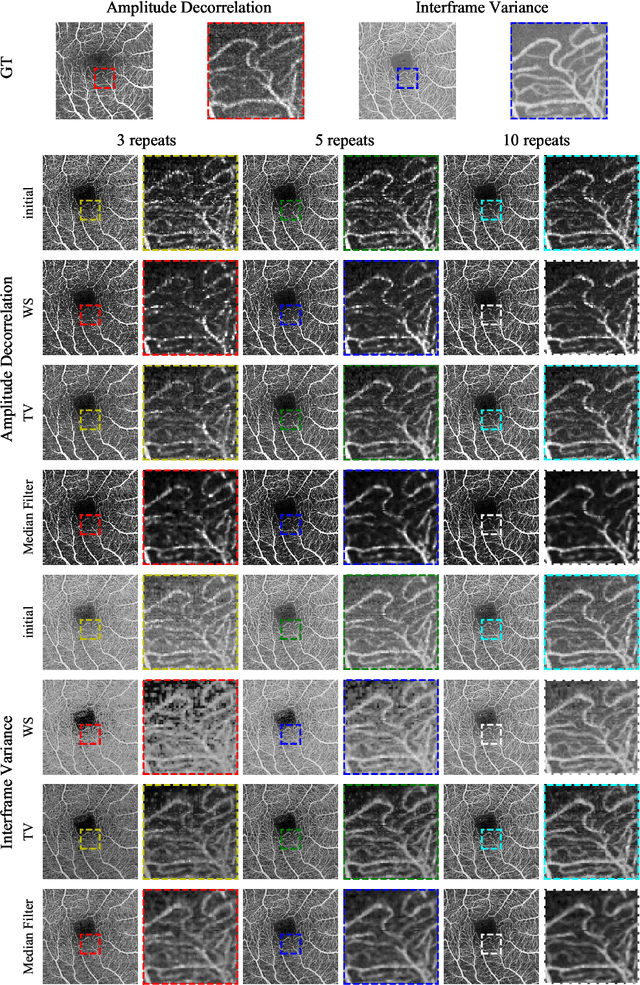
Abstract:Optical coherence tomography angiography (OCTA) is a novel and clinically promising imaging modality to image retinal and sub-retinal vasculature. Based on repeated optical coherence tomography (OCT) scans, intensity changes are observed over time and used to compute OCTA image data. OCTA data are prone to noise and artifacts caused by variations in flow speed and patient movement. We propose a novel iterative maximum a posteriori signal recovery algorithm in order to generate OCTA volumes with reduced noise and increased image quality. This algorithm is based on previous work on probabilistic OCTA signal models and maximum likelihood estimates. Reconstruction results using total variation minimization and wavelet shrinkage for regularization were compared against an OCTA ground truth volume, merged from six co-registered single OCTA volumes. The results show a significant improvement in peak signal-to-noise ratio and structural similarity. The presented algorithm brings together OCTA image generation and Bayesian statistics and can be developed into new OCTA image generation and denoising algorithms.
Efficient and high accuracy 3-D OCT angiography motion correction in pathology
Oct 14, 2020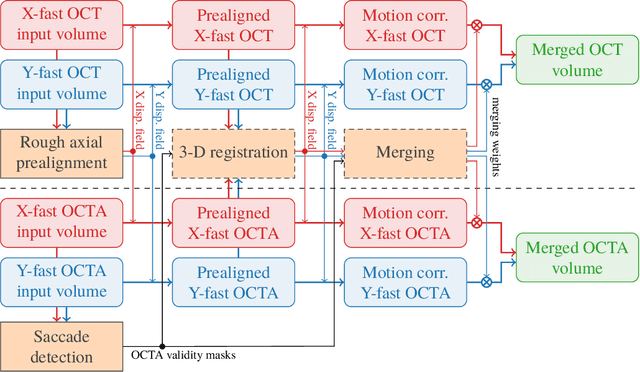
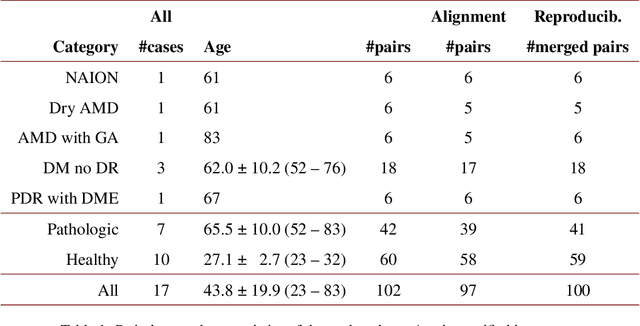
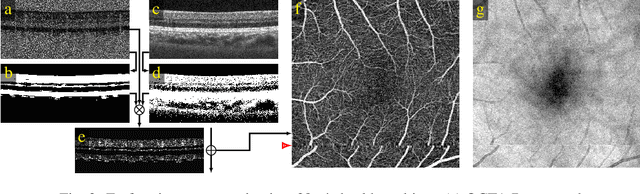
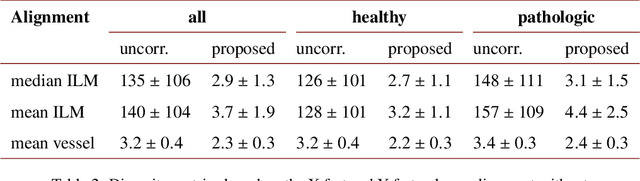
Abstract:We propose a novel method for non-rigid 3-D motion correction of orthogonally raster-scanned optical coherence tomography angiography volumes. This is the first approach that aligns predominantly axial structural features like retinal layers and transverse angiographic vascular features in a joint optimization. Combined with the use of orthogonal scans and favorization of kinematically more plausible displacements, the approach allows subpixel alignment and micrometer-scale distortion correction in all 3 dimensions. As no specific structures or layers are segmented, the approach is by design robust to pathologic changes. It is furthermore designed for highly parallel implementation and brief runtime, allowing its integration in clinical routine even for high density or wide-field scans. We evaluated the algorithm with metrics related to clinically relevant features in a large-scale quantitative evaluation based on 204 volumetric scans of 17 subjects including both a wide range of pathologies and healthy controls. Using this method, we achieve state-of-the-art axial performance and show significant advances in both transverse co-alignment and distortion correction, especially in the pathologic subgroup.
Deep OCT Angiography Image Generation for Motion Artifact Suppression
Jan 08, 2020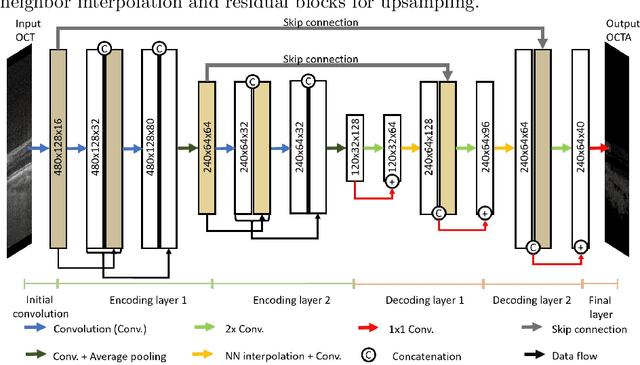
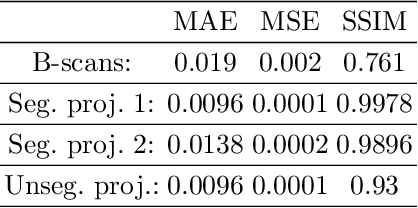
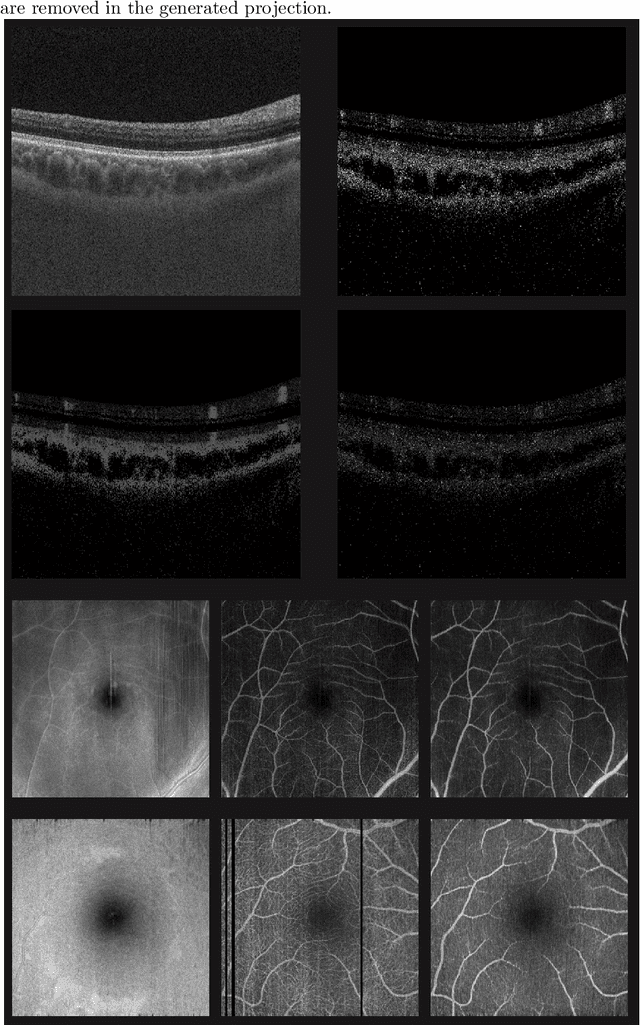
Abstract:Eye movements, blinking and other motion during the acquisition of optical coherence tomography (OCT) can lead to artifacts, when processed to OCT angiography (OCTA) images. Affected scans emerge as high intensity (white) or missing (black) regions, resulting in lost information. The aim of this research is to fill these gaps using a deep generative model for OCT to OCTA image translation relying on a single intact OCT scan. Therefore, a U-Net is trained to extract the angiographic information from OCT patches. At inference, a detection algorithm finds outlier OCTA scans based on their surroundings, which are then replaced by the trained network. We show that generative models can augment the missing scans. The augmented volumes could then be used for 3-D segmentation or increase the diagnostic value.
Lesson Learnt: Modularization of Deep Networks Allow Cross-Modality Reuse
Nov 05, 2019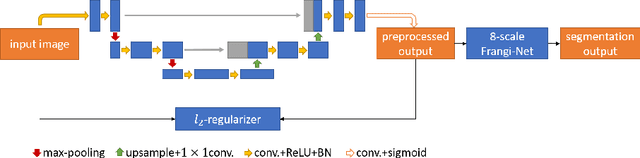

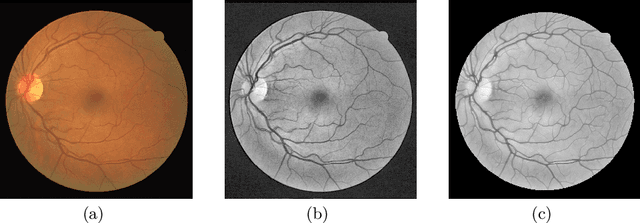
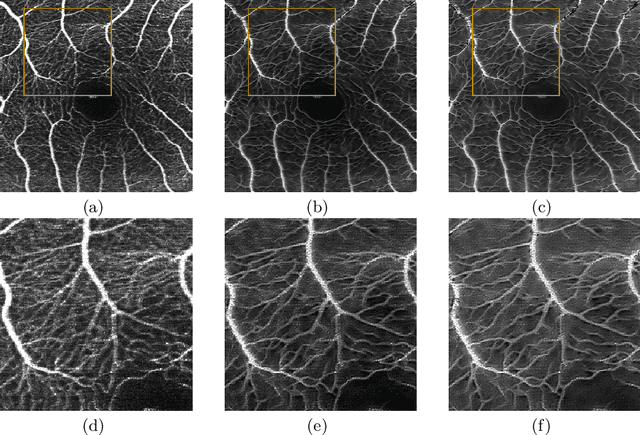
Abstract:Fundus photography and Optical Coherence Tomography Angiography (OCT-A) are two commonly used modalities in ophthalmic imaging. With the development of deep learning algorithms, fundus image processing, especially retinal vessel segmentation, has been extensively studied. Built upon the known operator theory, interpretable deep network pipelines with well-defined modules have been constructed on fundus images. In this work, we firstly train a modularized network pipeline for the task of retinal vessel segmentation on the fundus database DRIVE. The pretrained preprocessing module from the pipeline is then directly transferred onto OCT-A data for image quality enhancement without further fine-tuning. Output images show that the preprocessing net can balance the contrast, suppress noise and thereby produce vessel trees with improved connectivity in both image modalities. The visual impression is confirmed by an observer study with five OCT-A experts. Statistics of the grades by the experts indicate that the transferred module improves both the image quality and the diagnostic quality. Our work provides an example that modules within network pipelines that are built upon the known operator theory facilitate cross-modality reuse without additional training or transfer learning.
Temporal and Volumetric Denoising via Quantile Sparse Image (QuaSI) Prior in Optical Coherence Tomography and Beyond
Jul 05, 2018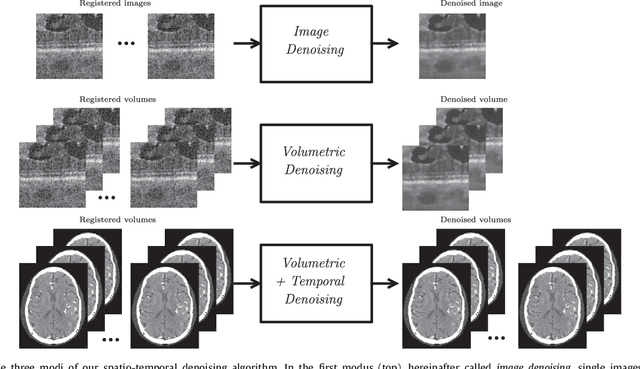
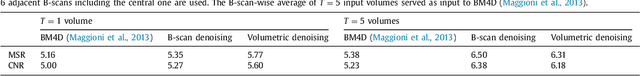
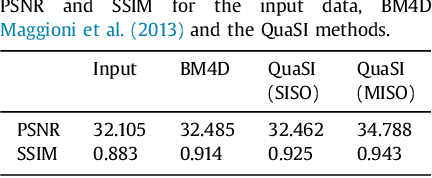
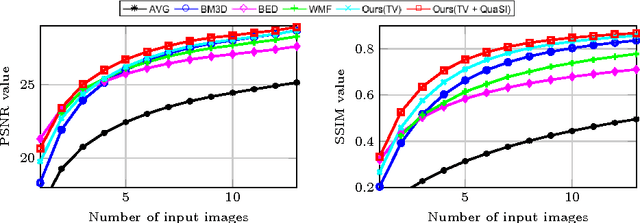
Abstract:This paper introduces an universal and structure-preserving regularization term, called quantile sparse image (QuaSI) prior. The prior is suitable for denoising images from various medical imaging modalities. We demonstrate its effectiveness on volumetric optical coherence tomography (OCT) and computed tomography (CT) data, which show different noise and image characteristics. OCT offers high-resolution scans of the human retina but is inherently impaired by speckle noise. CT on the other hand has a lower resolution and shows high-frequency noise. For the purpose of denoising, we propose a variational framework based on the QuaSI prior and a Huber data fidelity model that can handle 3-D and 3-D+t data. Efficient optimization is facilitated through the use of an alternating direction method of multipliers (ADMM) scheme and the linearization of the quantile filter. Experiments on multiple datasets emphasize the excellent performance of the proposed method.
QuaSI: Quantile Sparse Image Prior for Spatio-Temporal Denoising of Retinal OCT Data
Mar 08, 2017

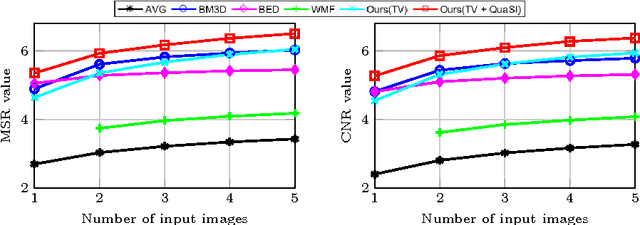
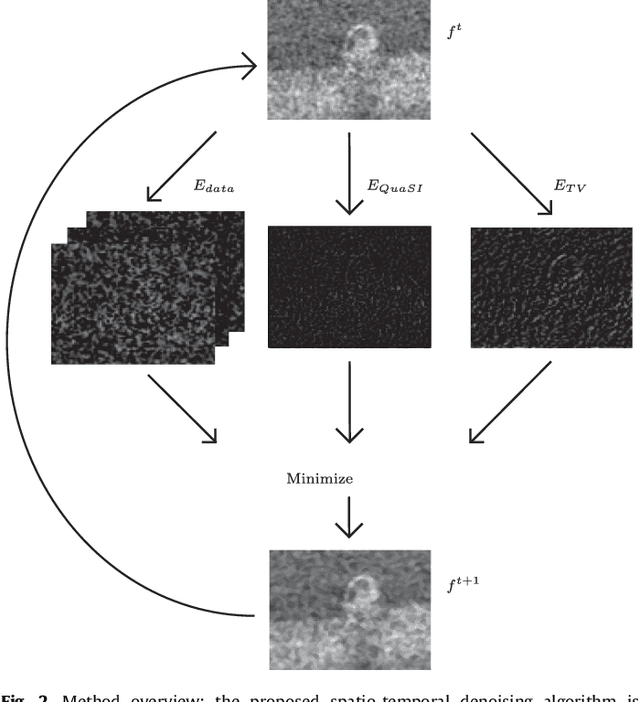
Abstract:Optical coherence tomography (OCT) enables high-resolution and non-invasive 3D imaging of the human retina but is inherently impaired by speckle noise. This paper introduces a spatio-temporal denoising algorithm for OCT data on a B-scan level using a novel quantile sparse image (QuaSI) prior. To remove speckle noise while preserving image structures of diagnostic relevance, we implement our QuaSI prior via median filter regularization coupled with a Huber data fidelity model in a variational approach. For efficient energy minimization, we develop an alternating direction method of multipliers (ADMM) scheme using a linearization of median filtering. Our spatio-temporal method can handle both, denoising of single B-scans and temporally consecutive B-scans, to gain volumetric OCT data with enhanced signal-to-noise ratio. Our algorithm based on 4 B-scans only achieved comparable performance to averaging 13 B-scans and outperformed other current denoising methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge