Jungeun Won
A Spatiotemporal Illumination Model for 3D Image Fusion in Optical Coherence Tomography
Feb 19, 2024Abstract:Optical coherence tomography (OCT) is a non-invasive, micrometer-scale imaging modality that has become a clinical standard in ophthalmology. By raster-scanning the retina, sequential cross-sectional image slices are acquired to generate volumetric data. In-vivo imaging suffers from discontinuities between slices that show up as motion and illumination artifacts. We present a new illumination model that exploits continuity in orthogonally raster-scanned volume data. Our novel spatiotemporal parametrization adheres to illumination continuity both temporally, along the imaged slices, as well as spatially, in the transverse directions. Yet, our formulation does not make inter-slice assumptions, which could have discontinuities. This is the first optimization of a 3D inverse model in an image reconstruction context in OCT. Evaluation in 68 volumes from eyes with pathology showed reduction of illumination artifacts in 88\% of the data, and only 6\% showed moderate residual illumination artifacts. The method enables the use of forward-warped motion corrected data, which is more accurate, and enables supersampling and advanced 3D image reconstruction in OCT.
Unsupervised detection of small hyperreflective features in ultrahigh resolution optical coherence tomography
Mar 26, 2023Abstract:Recent advances in optical coherence tomography such as the development of high speed ultrahigh resolution scanners and corresponding signal processing techniques may reveal new potential biomarkers in retinal diseases. Newly visible features are, for example, small hyperreflective specks in age-related macular degeneration. Identifying these new markers is crucial to investigate potential association with disease progression and treatment outcomes. Therefore, it is necessary to reliably detect these features in 3D volumetric scans. Because manual labeling of entire volumes is infeasible a need for automatic detection arises. Labeled datasets are often not publicly available and there are usually large variations in scan protocols and scanner types. Thus, this work focuses on an unsupervised approach that is based on local peak-detection and random walker segmentation to detect small features on each B-scan of the volume.
Retinal blood flow speed quantification at the capillary level using temporal autocorrelation fitting OCTA
Feb 22, 2023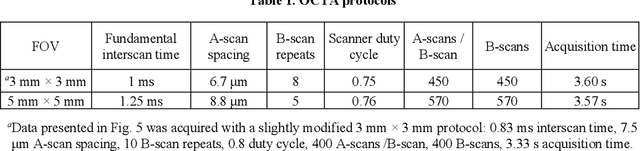
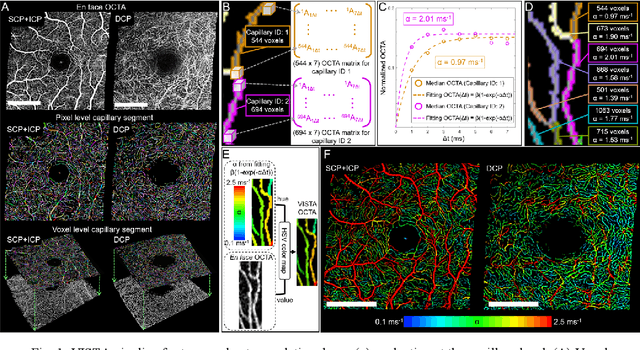
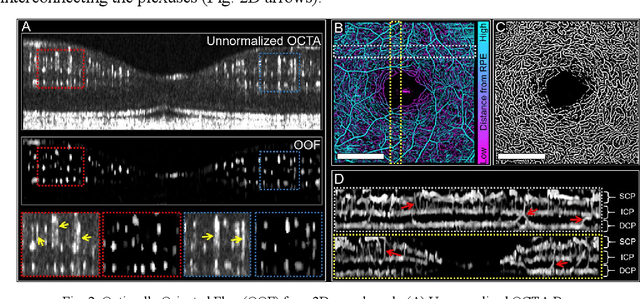
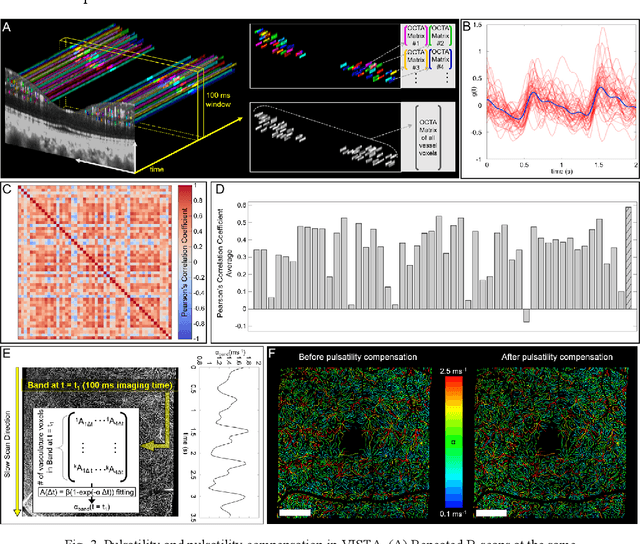
Abstract:Optical coherence tomography angiography (OCTA) can visualize vasculature structures, but provides limited information about the blood flow speeds. Here, we present a second generation variable interscan time analysis (VISTA) OCTA, which evaluates a quantitative surrogate marker for blood flow speed in vasculature. At the capillary level, spatially compiled OCTA and a simple temporal autocorrelation model, {\rho}({\tau}) = exp(-{\alpha}{\tau}), were used to evaluate a temporal autocorrelation decay constant, {\alpha}, as the blood flow speed marker. A 600 kHz A-scan rate swept-source provides short interscan time OCTA and fine A-scan spacing acquisition, while maintaining multi mm2 field of views for human retinal imaging. We demonstrate the cardiac pulsatility and repeatability of {\alpha} measured with VISTA. We show different {\alpha} for different retinal capillary plexuses in healthy eyes and present representative VISTA OCTA of eyes with diabetic retinopathy.
A Spatiotemporal Model for Precise and Efficient Fully-automatic 3D Motion Correction in OCT
Sep 15, 2022Abstract:Optical coherence tomography (OCT) is a micrometer-scale, volumetric imaging modality that has become a clinical standard in ophthalmology. OCT instruments image by raster-scanning a focused light spot across the retina, acquiring sequential cross-sectional images to generate volumetric data. Patient eye motion during the acquisition poses unique challenges: Non-rigid, discontinuous distortions can occur, leading to gaps in data and distorted topographic measurements. We present a new distortion model and a corresponding fully-automatic, reference-free optimization strategy for computational motion correction in orthogonally raster-scanned, retinal OCT volumes. Using a novel, domain-specific spatiotemporal parametrization of forward-warping displacements, eye motion can be corrected continuously for the first time. Parameter estimation with temporal regularization improves robustness and accuracy over previous spatial approaches. We correct each A-scan individually in 3D in a single mapping, including repeated acquisitions used in OCT angiography protocols. Specialized 3D forward image warping reduces median runtime to < 9 s, fast enough for clinical use. We present a quantitative evaluation on 18 subjects with ocular pathology and demonstrate accurate correction during microsaccades. Transverse correction is limited only by ocular tremor, whereas submicron repeatability is achieved axially (0.51 um median of medians), representing a dramatic improvement over previous work. This allows assessing longitudinal changes in focal retinal pathologies as a marker of disease progression or treatment response, and promises to enable multiple new capabilities such as supersampled/super-resolution volume reconstruction and analysis of pathological eye motion occuring in neurological diseases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge