Hiroyuki Takahashi
Department of Pathology, Jikei University School of Medicine, Tokyo, Japan
Net 582-Gb/s C-band and 4$\times$526-Gb/s O-band IMDD Transmis-sion Using Ultra-broadband InP-DHBT-based Electrical Mixer
Dec 23, 2025Abstract:We successfully transmitted a net 582-Gb/s probabilistically shaped PAM12 C-band signal over 11-km dispersion-shifted fibre and net 4$\times$526-Gb/s uniform PAM8 O-band signals over 2-km four-core fibre using a single-carrier 216-GBd IMDD system based on a 150-GHz bandwidth InP-DHBT electrical mixer and a thin-film lithium-niobate modulator.
389.3-Tb/s 1017-km C-band Transmission over Field-Installed 12-Coupled-Core Fiber Cable with >12-Tb/s Spatial MIMO Channels
Dec 10, 2025Abstract:We demonstrate 4.65-THz WDM/SDM transmission of 140-Gbaud PS-QAM signals over field-installed 12-coupled-core fiber cable with standard cladding diameter, achieving a record 0.455 Pb/s coupled-core capacity in a field environment. We also demonstrate 0.389 Pb/s over-1000-km transmission of spatial MIMO channels with >12 Tb/s/wavelength net bitrate.
133-Tbps 1040-km (13$\times$80 km) Lumped-Amplified Transmission Over 22 THz in S-to-U-Band Using Hybrid Multiband Repeater with PPLN-Based Optical Parametric Amplifiers and EDFAs
Dec 10, 2025Abstract:We demonstrated 22.05-THz four-band long-haul transmission with a S-to-U-band lumped repeater consisting of PPLN-based optical parametric amplifiers and EDFAs over an 80-km-span SMF link. The achieved net bitrate was 133.06 Tbps at 1040 km with the 25.5-dBm fibre launch power designed by accounting for ISRS.
Future frame prediction in chest cine MR imaging using the PCA respiratory motion model and dynamically trained recurrent neural networks
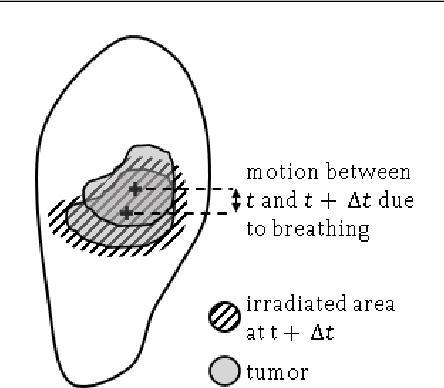
Oct 08, 2024Abstract:Lung radiotherapy treatment systems are subject to a latency that leads to uncertainty in the estimated tumor location and high irradiation of healthy tissue. This work addresses future frame prediction in chest dynamic MRI sequences to compensate for that delay using RNNs trained with online learning algorithms. The latter enable networks to mitigate irregular movements, as they update synaptic weights with each new training example. Experiments were conducted using four publicly available 2D thoracic cine-MRI sequences. PCA decomposes the time-varying deformation vector field (DVF), computed with the Lucas-Kanade optical flow algorithm, into static deformation fields and low-dimensional time-dependent weights. We compare various algorithms to forecast the latter: linear regression, least mean squares (LMS), and RNNs trained with real-time recurrent learning (RTRL), unbiased online recurrent optimization, decoupled neural interfaces and sparse 1-step approximation (SnAp-1). That enables estimating the future DVFs and, in turn, the next frames by warping the initial image. Linear regression led to the lowest mean DVF error at a horizon h = 0.32s (the time interval in advance for which the prediction is made), equal to 1.30mm, followed by SnAp-1 and RTRL, whose error increased from 1.37mm to 1.44mm as h increased from 0.62s to 2.20s. Similarly, the structural similarity index measure (SSIM) of LMS decreased from 0.904 to 0.898 as h increased from 0.31s to 1.57s and was the highest among the algorithms compared for the latter horizons. SnAp-1 attained the highest SSIM for h $\geq$ 1.88s, with values of less than 0.898. The predicted images look similar to the original ones, and the highest errors occurred at challenging areas such as the diaphragm boundary at the end-of-inhale phase, where motion variability is more prominent, and regions where out-of-plane motion was more prevalent.
Respiratory motion forecasting with online learning of recurrent neural networks for safety enhancement in externally guided radiotherapy
Mar 03, 2024



Abstract:In lung radiotherapy, infrared cameras can record the location of reflective objects on the chest to infer the position of the tumor moving due to breathing, but treatment system latencies hinder radiation beam precision. Real-time recurrent learning (RTRL), is a potential solution as it can learn patterns within non-stationary respiratory data but has high complexity. This study assesses the capabilities of resource-efficient online RNN algorithms, namely unbiased online recurrent optimization (UORO), sparse-1 step approximation (SnAp-1), and decoupled neural interfaces (DNI) to forecast respiratory motion during radiotherapy treatment accurately. We use time series containing the 3D position of external markers on the chest of healthy subjects. We propose efficient implementations for SnAp-1 and DNI based on compression of the influence and immediate Jacobian matrices and an accurate update of the linear coefficients used in credit assignment estimation, respectively. The original sampling frequency was 10Hz; we performed resampling at 3.33Hz and 30Hz. We use UORO, SnAp-1, and DNI to forecast each marker's 3D position with horizons (the time interval in advance for which the prediction is made) h<=2.1s and compare them with RTRL, least mean squares, and linear regression. RNNs trained online achieved similar or better accuracy than most previous works using larger training databases and deep learning, even though we used only the first minute of each sequence to predict motion within that exact sequence. SnAp-1 had the lowest normalized root mean square errors (nRMSE) averaged over the horizon values considered, equal to 0.335 and 0.157, at 3.33Hz and 10.0Hz, respectively. Similarly, UORO had the highest accuracy at 30Hz, with an nRMSE of 0.0897. DNI's inference time, equal to 6.8ms per time step at 30Hz (Intel Core i7-13700 CPU), was the lowest among the RNN methods examined.
Unsupervised detection of small hyperreflective features in ultrahigh resolution optical coherence tomography
Mar 26, 2023Abstract:Recent advances in optical coherence tomography such as the development of high speed ultrahigh resolution scanners and corresponding signal processing techniques may reveal new potential biomarkers in retinal diseases. Newly visible features are, for example, small hyperreflective specks in age-related macular degeneration. Identifying these new markers is crucial to investigate potential association with disease progression and treatment outcomes. Therefore, it is necessary to reliably detect these features in 3D volumetric scans. Because manual labeling of entire volumes is infeasible a need for automatic detection arises. Labeled datasets are often not publicly available and there are usually large variations in scan protocols and scanner types. Thus, this work focuses on an unsupervised approach that is based on local peak-detection and random walker segmentation to detect small features on each B-scan of the volume.
Retinal blood flow speed quantification at the capillary level using temporal autocorrelation fitting OCTA
Feb 22, 2023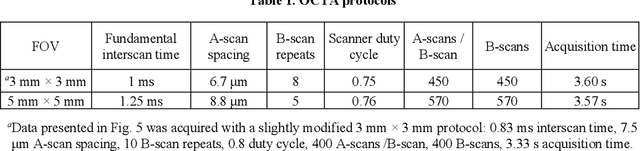
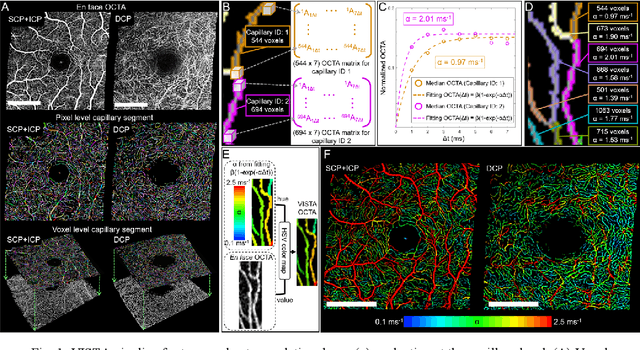
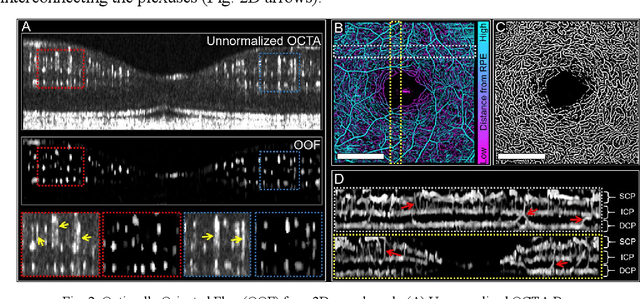
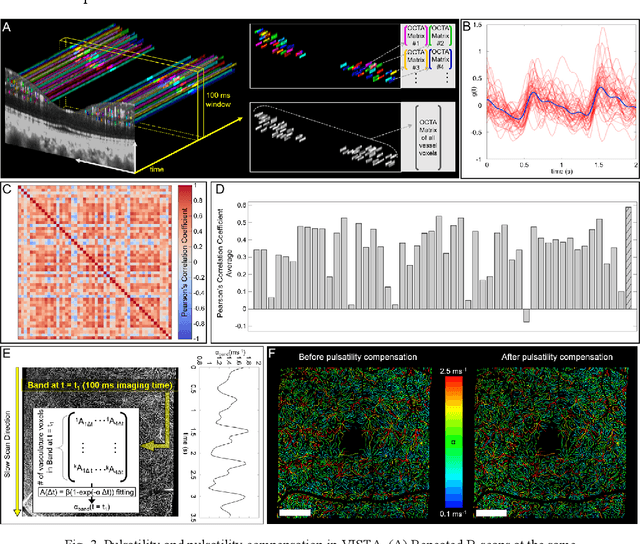
Abstract:Optical coherence tomography angiography (OCTA) can visualize vasculature structures, but provides limited information about the blood flow speeds. Here, we present a second generation variable interscan time analysis (VISTA) OCTA, which evaluates a quantitative surrogate marker for blood flow speed in vasculature. At the capillary level, spatially compiled OCTA and a simple temporal autocorrelation model, {\rho}({\tau}) = exp(-{\alpha}{\tau}), were used to evaluate a temporal autocorrelation decay constant, {\alpha}, as the blood flow speed marker. A 600 kHz A-scan rate swept-source provides short interscan time OCTA and fine A-scan spacing acquisition, while maintaining multi mm2 field of views for human retinal imaging. We demonstrate the cardiac pulsatility and repeatability of {\alpha} measured with VISTA. We show different {\alpha} for different retinal capillary plexuses in healthy eyes and present representative VISTA OCTA of eyes with diabetic retinopathy.
Prediction of the Position of External Markers Using a Recurrent Neural Network Trained With Unbiased Online Recurrent Optimization for Safe Lung Cancer Radiotherapy
Jun 02, 2021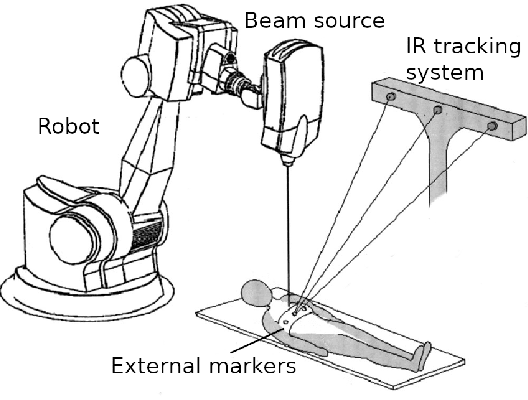
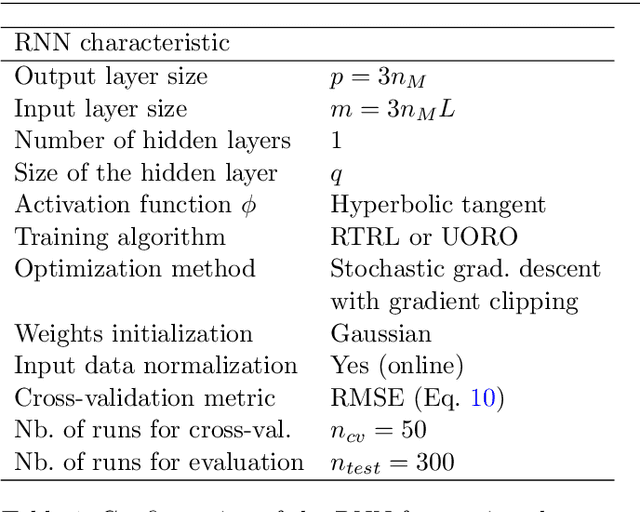
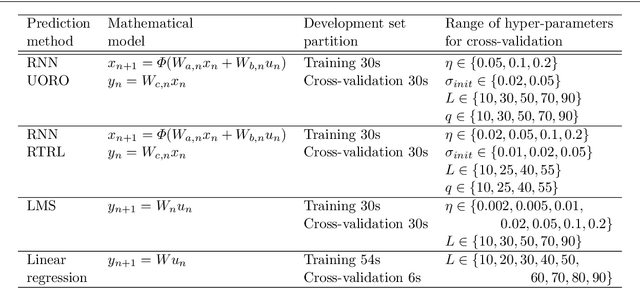
Abstract:During lung cancer radiotherapy, the position of infrared reflective objects on the chest can be recorded to estimate the tumor location. However, radiotherapy systems usually have a latency inherent to robot control limitations that impedes the radiation delivery precision. Not taking this phenomenon into account may cause unwanted damage to healthy tissues and lead to side effects such as radiation pneumonitis. In this research, we use nine observation records of the three-dimensional position of three external markers on the chest and abdomen of healthy individuals breathing during intervals from 73s to 222s. The sampling frequency is equal to 10Hz and the amplitudes of the recorded trajectories range from 6mm to 40mm in the superior-inferior direction. We forecast the location of each marker simultaneously with a horizon value (the time interval in advance for which the prediction is made) between 0.1s and 2.0s, using a recurrent neural network (RNN) trained with unbiased online recurrent optimization (UORO). We compare its performance with an RNN trained with real-time recurrent learning, least mean squares (LMS), and offline linear regression. Training and cross-validation are performed during the first minute of each sequence. On average, UORO achieves the lowest root-mean-square (RMS) and maximum error, equal respectively to 1.3mm and 8.8mm, with a prediction time per time step lower than 2.8ms (Dell Intel core i9-9900K 3.60Ghz). Linear regression has the lowest RMS error for the horizon values 0.1s and 0.2s, followed by LMS for horizon values between 0.3s and 0.5s, and UORO for horizon values greater than 0.6s.
Pathologist-Level Grading of Prostate Biopsies with Artificial Intelligence
Jul 02, 2019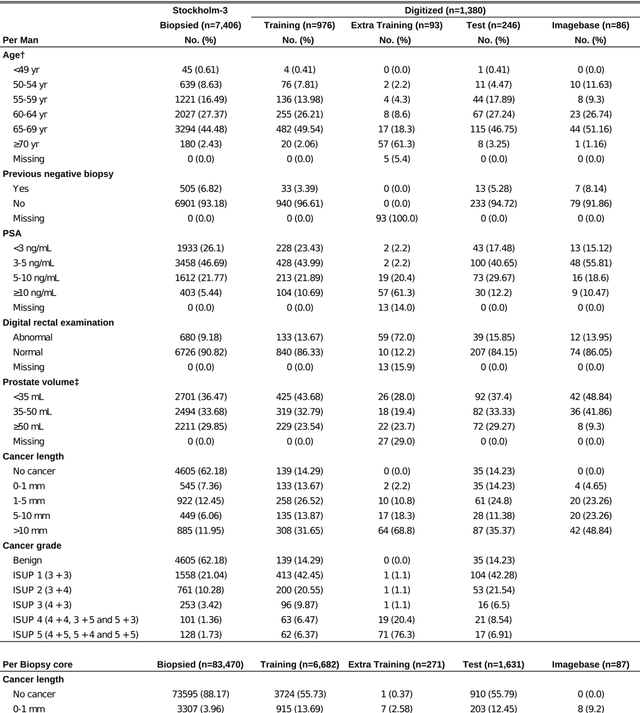
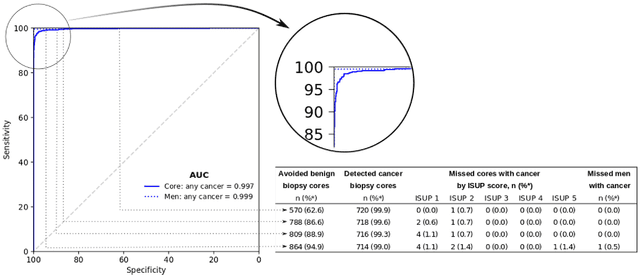
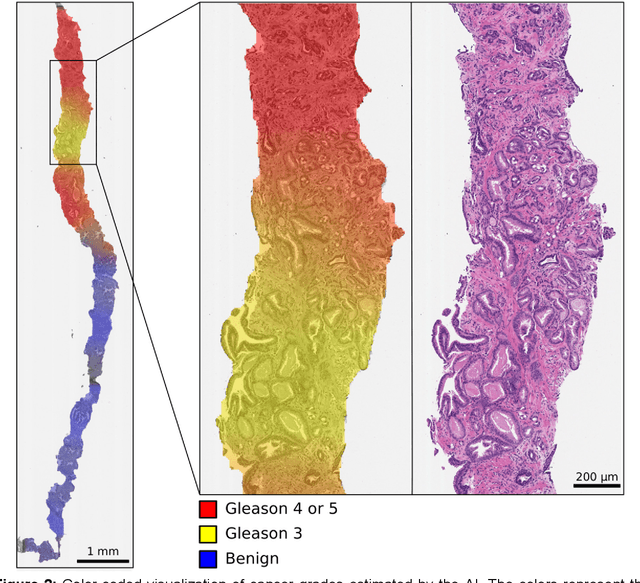
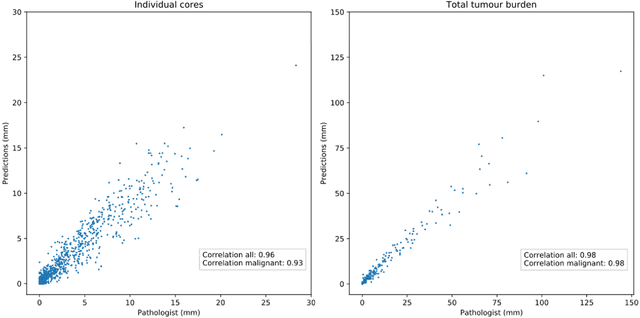
Abstract:Background: An increasing volume of prostate biopsies and a world-wide shortage of uro-pathologists puts a strain on pathology departments. Additionally, the high intra- and inter-observer variability in grading can result in over- and undertreatment of prostate cancer. Artificial intelligence (AI) methods may alleviate these problems by assisting pathologists to reduce workload and harmonize grading. Methods: We digitized 6,682 needle biopsies from 976 participants in the population based STHLM3 diagnostic study to train deep neural networks for assessing prostate biopsies. The networks were evaluated by predicting the presence, extent, and Gleason grade of malignant tissue for an independent test set comprising 1,631 biopsies from 245 men. We additionally evaluated grading performance on 87 biopsies individually graded by 23 experienced urological pathologists from the International Society of Urological Pathology. We assessed discriminatory performance by receiver operating characteristics (ROC) and tumor extent predictions by correlating predicted millimeter cancer length against measurements by the reporting pathologist. We quantified the concordance between grades assigned by the AI and the expert urological pathologists using Cohen's kappa. Results: The performance of the AI to detect and grade cancer in prostate needle biopsy samples was comparable to that of international experts in prostate pathology. The AI achieved an area under the ROC curve of 0.997 for distinguishing between benign and malignant biopsy cores, and 0.999 for distinguishing between men with or without prostate cancer. The correlation between millimeter cancer predicted by the AI and assigned by the reporting pathologist was 0.96. For assigning Gleason grades, the AI achieved an average pairwise kappa of 0.62. This was within the range of the corresponding values for the expert pathologists (0.60 to 0.73).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge