Joumana Ghosn
Superintelligent Agents Pose Catastrophic Risks: Can Scientist AI Offer a Safer Path?
Feb 21, 2025Abstract:The leading AI companies are increasingly focused on building generalist AI agents -- systems that can autonomously plan, act, and pursue goals across almost all tasks that humans can perform. Despite how useful these systems might be, unchecked AI agency poses significant risks to public safety and security, ranging from misuse by malicious actors to a potentially irreversible loss of human control. We discuss how these risks arise from current AI training methods. Indeed, various scenarios and experiments have demonstrated the possibility of AI agents engaging in deception or pursuing goals that were not specified by human operators and that conflict with human interests, such as self-preservation. Following the precautionary principle, we see a strong need for safer, yet still useful, alternatives to the current agency-driven trajectory. Accordingly, we propose as a core building block for further advances the development of a non-agentic AI system that is trustworthy and safe by design, which we call Scientist AI. This system is designed to explain the world from observations, as opposed to taking actions in it to imitate or please humans. It comprises a world model that generates theories to explain data and a question-answering inference machine. Both components operate with an explicit notion of uncertainty to mitigate the risks of overconfident predictions. In light of these considerations, a Scientist AI could be used to assist human researchers in accelerating scientific progress, including in AI safety. In particular, our system can be employed as a guardrail against AI agents that might be created despite the risks involved. Ultimately, focusing on non-agentic AI may enable the benefits of AI innovation while avoiding the risks associated with the current trajectory. We hope these arguments will motivate researchers, developers, and policymakers to favor this safer path.
Towards Trustworthy Automatic Diagnosis Systems by Emulating Doctors' Reasoning with Deep Reinforcement Learning
Oct 13, 2022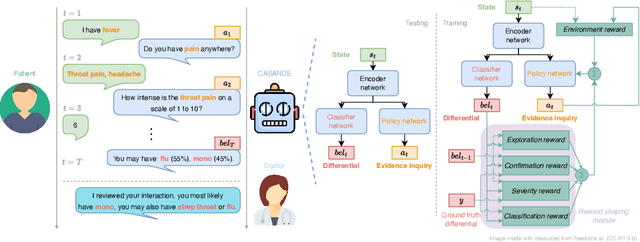
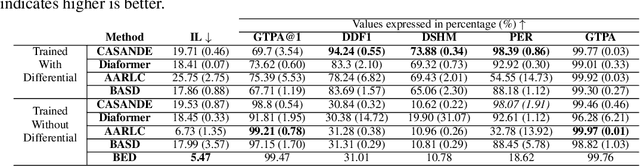
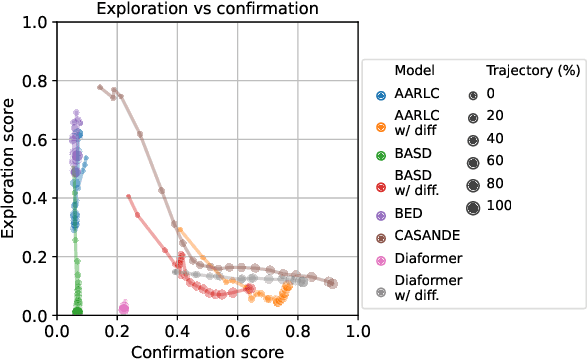

Abstract:The automation of the medical evidence acquisition and diagnosis process has recently attracted increasing attention in order to reduce the workload of doctors and democratize access to medical care. However, most works proposed in the machine learning literature focus solely on improving the prediction accuracy of a patient's pathology. We argue that this objective is insufficient to ensure doctors' acceptability of such systems. In their initial interaction with patients, doctors do not only focus on identifying the pathology a patient is suffering from; they instead generate a differential diagnosis (in the form of a short list of plausible diseases) because the medical evidence collected from patients is often insufficient to establish a final diagnosis. Moreover, doctors explicitly explore severe pathologies before potentially ruling them out from the differential, especially in acute care settings. Finally, for doctors to trust a system's recommendations, they need to understand how the gathered evidences led to the predicted diseases. In particular, interactions between a system and a patient need to emulate the reasoning of doctors. We therefore propose to model the evidence acquisition and automatic diagnosis tasks using a deep reinforcement learning framework that considers three essential aspects of a doctor's reasoning, namely generating a differential diagnosis using an exploration-confirmation approach while prioritizing severe pathologies. We propose metrics for evaluating interaction quality based on these three aspects. We show that our approach performs better than existing models while maintaining competitive pathology prediction accuracy.
DDXPlus: A new Dataset for Medical Automatic Diagnosis
May 18, 2022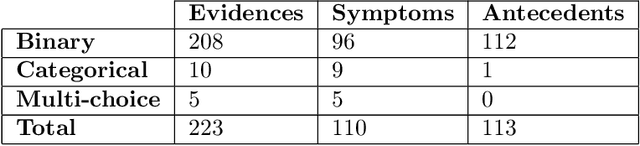
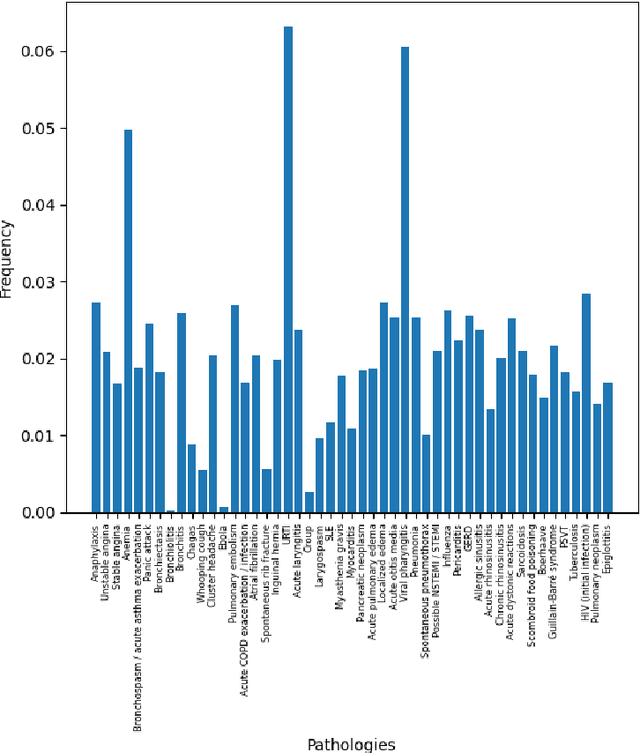
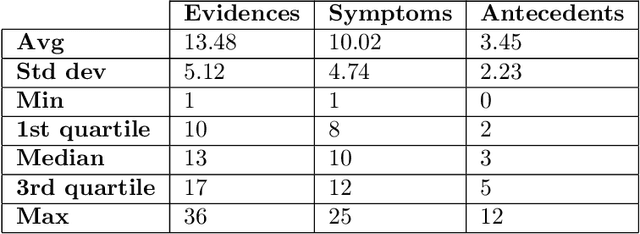
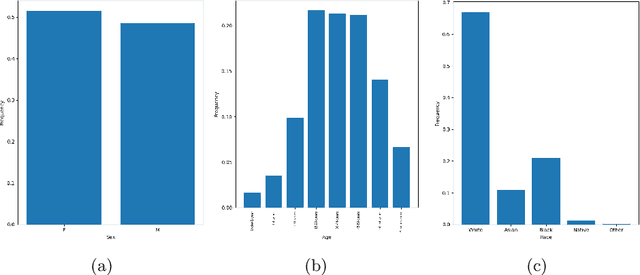
Abstract:There has been rapidly growing interests in Automatic Diagnosis (AD) and Automatic Symptom Detection (ASD) systems in the machine learning research literature, aiming to assist doctors in telemedicine services. These systems are designed to interact with patients, collect evidence relevant to their concerns, and make predictions about the underlying diseases. Doctors would review the interaction, including the evidence and the predictions, before making their final decisions. Despite the recent progress, an important piece of doctors' interactions with patients is missing in the design of AD and ASD systems, namely the differential diagnosis. Its absence is largely due to the lack of datasets that include such information for models to train on. In this work, we present a large-scale synthetic dataset that includes a differential diagnosis, along with the ground truth pathology, for each patient. In addition, this dataset includes more pathologies, as well as types of symtoms and antecedents. As a proof-of-concept, we extend several existing AD and ASD systems to incorporate differential diagnosis, and provide empirical evidence that using differentials in training signals is essential for such systems to learn to predict differentials. Dataset available at https://github.com/bruzwen/ddxplus
COVI-AgentSim: an Agent-based Model for Evaluating Methods of Digital Contact Tracing
Oct 30, 2020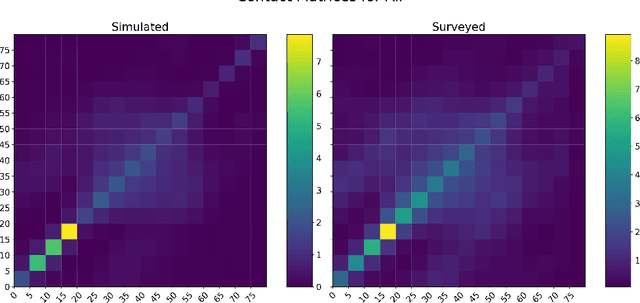
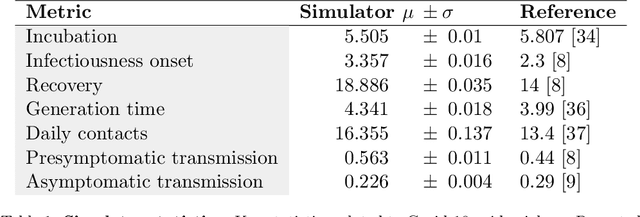
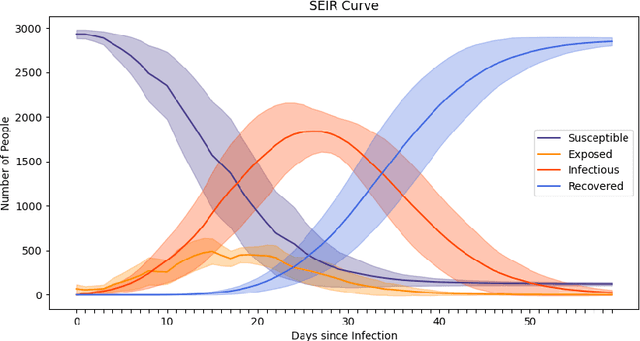
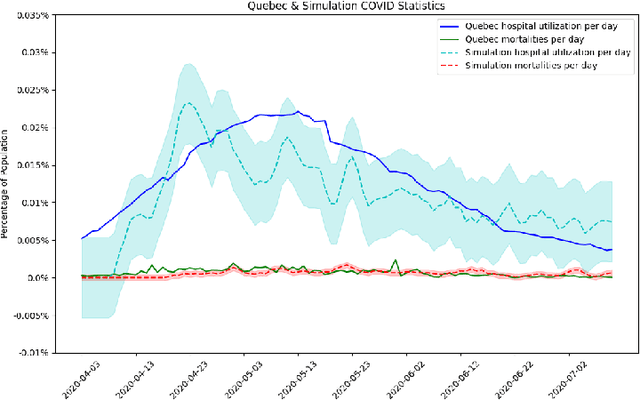
Abstract:The rapid global spread of COVID-19 has led to an unprecedented demand for effective methods to mitigate the spread of the disease, and various digital contact tracing (DCT) methods have emerged as a component of the solution. In order to make informed public health choices, there is a need for tools which allow evaluation and comparison of DCT methods. We introduce an agent-based compartmental simulator we call COVI-AgentSim, integrating detailed consideration of virology, disease progression, social contact networks, and mobility patterns, based on parameters derived from empirical research. We verify by comparing to real data that COVI-AgentSim is able to reproduce realistic COVID-19 spread dynamics, and perform a sensitivity analysis to verify that the relative performance of contact tracing methods are consistent across a range of settings. We use COVI-AgentSim to perform cost-benefit analyses comparing no DCT to: 1) standard binary contact tracing (BCT) that assigns binary recommendations based on binary test results; and 2) a rule-based method for feature-based contact tracing (FCT) that assigns a graded level of recommendation based on diverse individual features. We find all DCT methods consistently reduce the spread of the disease, and that the advantage of FCT over BCT is maintained over a wide range of adoption rates. Feature-based methods of contact tracing avert more disability-adjusted life years (DALYs) per socioeconomic cost (measured by productive hours lost). Our results suggest any DCT method can help save lives, support re-opening of economies, and prevent second-wave outbreaks, and that FCT methods are a promising direction for enriching BCT using self-reported symptoms, yielding earlier warning signals and a significantly reduced spread of the virus per socioeconomic cost.
Predicting Infectiousness for Proactive Contact Tracing
Oct 23, 2020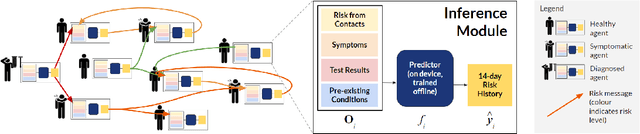
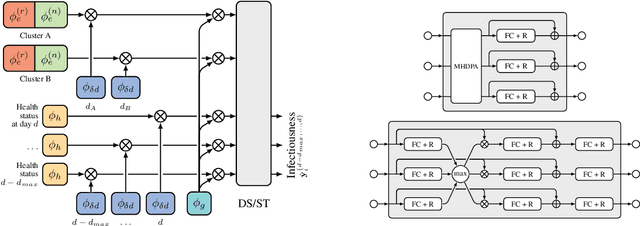
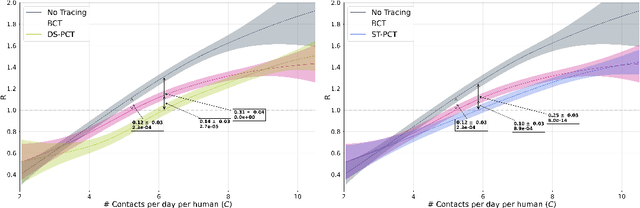
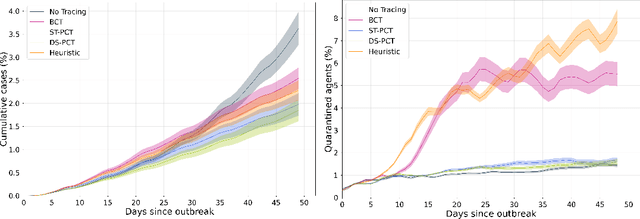
Abstract:The COVID-19 pandemic has spread rapidly worldwide, overwhelming manual contact tracing in many countries and resulting in widespread lockdowns for emergency containment. Large-scale digital contact tracing (DCT) has emerged as a potential solution to resume economic and social activity while minimizing spread of the virus. Various DCT methods have been proposed, each making trade-offs between privacy, mobility restrictions, and public health. The most common approach, binary contact tracing (BCT), models infection as a binary event, informed only by an individual's test results, with corresponding binary recommendations that either all or none of the individual's contacts quarantine. BCT ignores the inherent uncertainty in contacts and the infection process, which could be used to tailor messaging to high-risk individuals, and prompt proactive testing or earlier warnings. It also does not make use of observations such as symptoms or pre-existing medical conditions, which could be used to make more accurate infectiousness predictions. In this paper, we use a recently-proposed COVID-19 epidemiological simulator to develop and test methods that can be deployed to a smartphone to locally and proactively predict an individual's infectiousness (risk of infecting others) based on their contact history and other information, while respecting strong privacy constraints. Predictions are used to provide personalized recommendations to the individual via an app, as well as to send anonymized messages to the individual's contacts, who use this information to better predict their own infectiousness, an approach we call proactive contact tracing (PCT). We find a deep-learning based PCT method which improves over BCT for equivalent average mobility, suggesting PCT could help in safe re-opening and second-wave prevention.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge