John A. Onofrey
Equi-ViT: Rotational Equivariant Vision Transformer for Robust Histopathology Analysis
Jan 14, 2026Abstract:Vision Transformers (ViTs) have gained rapid adoption in computational pathology for their ability to model long-range dependencies through self-attention, addressing the limitations of convolutional neural networks that excel at local pattern capture but struggle with global contextual reasoning. Recent pathology-specific foundation models have further advanced performance by leveraging large-scale pretraining. However, standard ViTs remain inherently non-equivariant to transformations such as rotations and reflections, which are ubiquitous variations in histopathology imaging. To address this limitation, we propose Equi-ViT, which integrates an equivariant convolution kernel into the patch embedding stage of a ViT architecture, imparting built-in rotational equivariance to learned representations. Equi-ViT achieves superior rotation-consistent patch embeddings and stable classification performance across image orientations. Our results on a public colorectal cancer dataset demonstrate that incorporating equivariant patch embedding enhances data efficiency and robustness, suggesting that equivariant transformers could potentially serve as more generalizable backbones for the application of ViT in histopathology, such as digital pathology foundation models.
PET Head Motion Estimation Using Supervised Deep Learning with Attention
Oct 14, 2025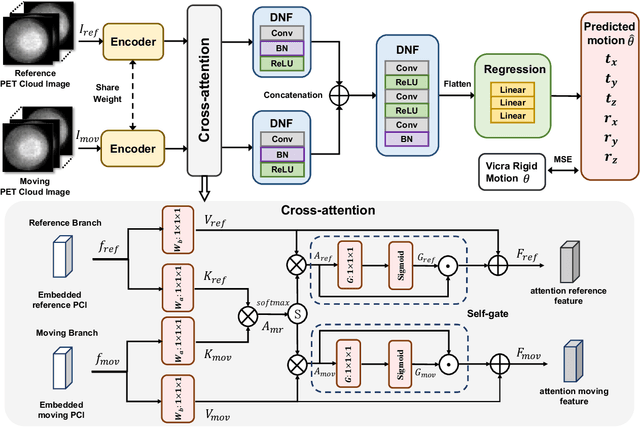
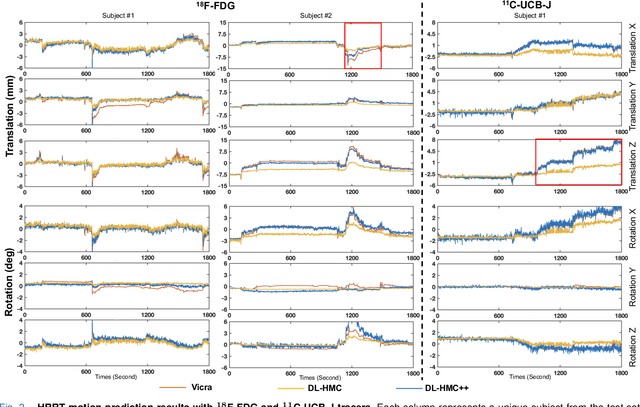
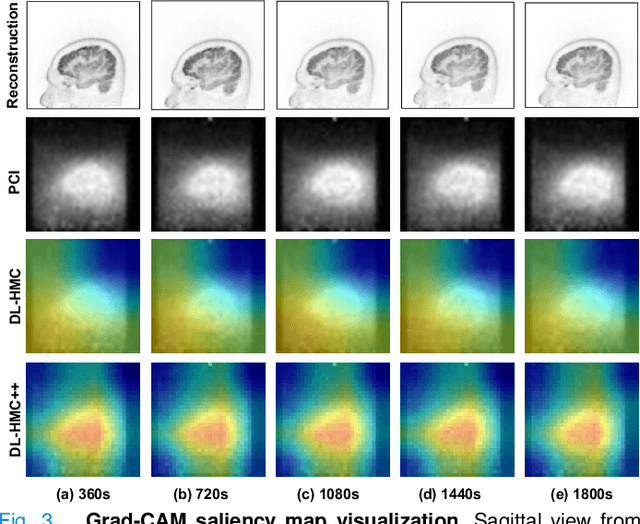
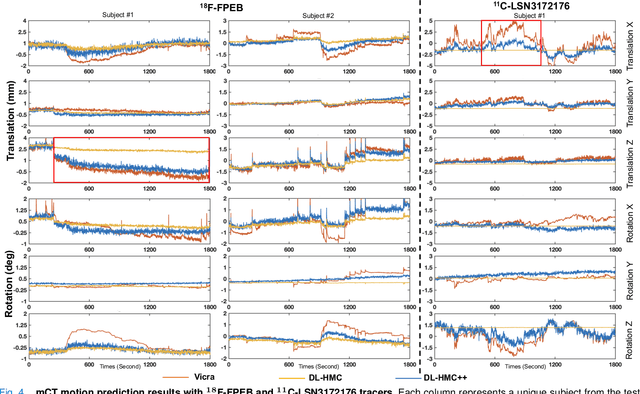
Abstract:Head movement poses a significant challenge in brain positron emission tomography (PET) imaging, resulting in image artifacts and tracer uptake quantification inaccuracies. Effective head motion estimation and correction are crucial for precise quantitative image analysis and accurate diagnosis of neurological disorders. Hardware-based motion tracking (HMT) has limited applicability in real-world clinical practice. To overcome this limitation, we propose a deep-learning head motion correction approach with cross-attention (DL-HMC++) to predict rigid head motion from one-second 3D PET raw data. DL-HMC++ is trained in a supervised manner by leveraging existing dynamic PET scans with gold-standard motion measurements from external HMT. We evaluate DL-HMC++ on two PET scanners (HRRT and mCT) and four radiotracers (18F-FDG, 18F-FPEB, 11C-UCB-J, and 11C-LSN3172176) to demonstrate the effectiveness and generalization of the approach in large cohort PET studies. Quantitative and qualitative results demonstrate that DL-HMC++ consistently outperforms state-of-the-art data-driven motion estimation methods, producing motion-free images with clear delineation of brain structures and reduced motion artifacts that are indistinguishable from gold-standard HMT. Brain region of interest standard uptake value analysis exhibits average difference ratios between DL-HMC++ and gold-standard HMT to be 1.2 plus-minus 0.5% for HRRT and 0.5 plus-minus 0.2% for mCT. DL-HMC++ demonstrates the potential for data-driven PET head motion correction to remove the burden of HMT, making motion correction accessible to clinical populations beyond research settings. The code is available at https://github.com/maxxxxxxcai/DL-HMC-TMI.
Equivariant Imaging Biomarkers for Robust Unsupervised Segmentation of Histopathology
May 08, 2025Abstract:Histopathology evaluation of tissue specimens through microscopic examination is essential for accurate disease diagnosis and prognosis. However, traditional manual analysis by specially trained pathologists is time-consuming, labor-intensive, cost-inefficient, and prone to inter-rater variability, potentially affecting diagnostic consistency and accuracy. As digital pathology images continue to proliferate, there is a pressing need for automated analysis to address these challenges. Recent advancements in artificial intelligence-based tools such as machine learning (ML) models, have significantly enhanced the precision and efficiency of analyzing histopathological slides. However, despite their impressive performance, ML models are invariant only to translation, lacking invariance to rotation and reflection. This limitation restricts their ability to generalize effectively, particularly in histopathology, where images intrinsically lack meaningful orientation. In this study, we develop robust, equivariant histopathological biomarkers through a novel symmetric convolutional kernel via unsupervised segmentation. The approach is validated using prostate tissue micro-array (TMA) images from 50 patients in the Gleason 2019 Challenge public dataset. The biomarkers extracted through this approach demonstrate enhanced robustness and generalizability against rotation compared to models using standard convolution kernels, holding promise for enhancing the accuracy, consistency, and robustness of ML models in digital pathology. Ultimately, this work aims to improve diagnostic and prognostic capabilities of histopathology beyond prostate cancer through equivariant imaging.
GMR-Conv: An Efficient Rotation and Reflection Equivariant Convolution Kernel Using Gaussian Mixture Rings
Apr 03, 2025Abstract:Symmetry, where certain features remain invariant under geometric transformations, can often serve as a powerful prior in designing convolutional neural networks (CNNs). While conventional CNNs inherently support translational equivariance, extending this property to rotation and reflection has proven challenging, often forcing a compromise between equivariance, efficiency, and information loss. In this work, we introduce Gaussian Mixture Ring Convolution (GMR-Conv), an efficient convolution kernel that smooths radial symmetry using a mixture of Gaussian-weighted rings. This design mitigates discretization errors of circular kernels, thereby preserving robust rotation and reflection equivariance without incurring computational overhead. We further optimize both the space and speed efficiency of GMR-Conv via a novel parameterization and computation strategy, allowing larger kernels at an acceptable cost. Extensive experiments on eight classification and one segmentation datasets demonstrate that GMR-Conv not only matches conventional CNNs' performance but can also surpass it in applications with orientation-less data. GMR-Conv is also proven to be more robust and efficient than the state-of-the-art equivariant learning methods. Our work provides inspiring empirical evidence that carefully applied radial symmetry can alleviate the challenges of information loss, marking a promising advance in equivariant network architectures. The code is available at https://github.com/XYPB/GMR-Conv.
Improved Vessel Segmentation with Symmetric Rotation-Equivariant U-Net
Jan 24, 2025Abstract:Automated segmentation plays a pivotal role in medical image analysis and computer-assisted interventions. Despite the promising performance of existing methods based on convolutional neural networks (CNNs), they neglect useful equivariant properties for images, such as rotational and reflection equivariance. This limitation can decrease performance and lead to inconsistent predictions, especially in applications like vessel segmentation where explicit orientation is absent. While existing equivariant learning approaches attempt to mitigate these issues, they substantially increase learning cost, model size, or both. To overcome these challenges, we propose a novel application of an efficient symmetric rotation-equivariant (SRE) convolutional (SRE-Conv) kernel implementation to the U-Net architecture, to learn rotation and reflection-equivariant features, while also reducing the model size dramatically. We validate the effectiveness of our method through improved segmentation performance on retina vessel fundus imaging. Our proposed SRE U-Net not only significantly surpasses standard U-Net in handling rotated images, but also outperforms existing equivariant learning methods and does so with a reduced number of trainable parameters and smaller memory cost. The code is available at https://github.com/OnofreyLab/sre_conv_segm_isbi2025.
Enhancing Uncertainty Estimation in Semantic Segmentation via Monte-Carlo Frequency Dropout
Jan 20, 2025



Abstract:Monte-Carlo (MC) Dropout provides a practical solution for estimating predictive distributions in deterministic neural networks. Traditional dropout, applied within the signal space, may fail to account for frequency-related noise common in medical imaging, leading to biased predictive estimates. A novel approach extends Dropout to the frequency domain, allowing stochastic attenuation of signal frequencies during inference. This creates diverse global textural variations in feature maps while preserving structural integrity -- a factor we hypothesize and empirically show is contributing to accurately estimating uncertainties in semantic segmentation. We evaluated traditional MC-Dropout and the MC-frequency Dropout in three segmentation tasks involving different imaging modalities: (i) prostate zones in biparametric MRI, (ii) liver tumors in contrast-enhanced CT, and (iii) lungs in chest X-ray scans. Our results show that MC-Frequency Dropout improves calibration, convergence, and semantic uncertainty, thereby improving prediction scrutiny, boundary delineation, and has the potential to enhance medical decision-making.
SRE-Conv: Symmetric Rotation Equivariant Convolution for Biomedical Image Classification
Jan 16, 2025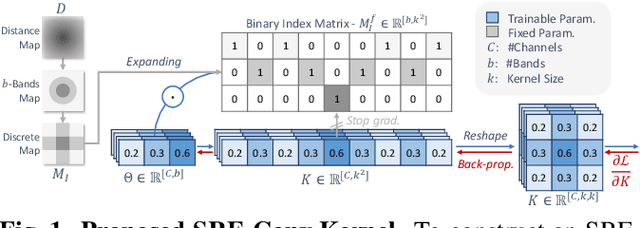
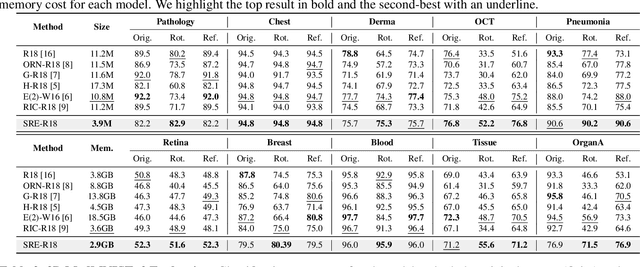
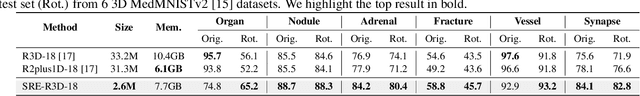
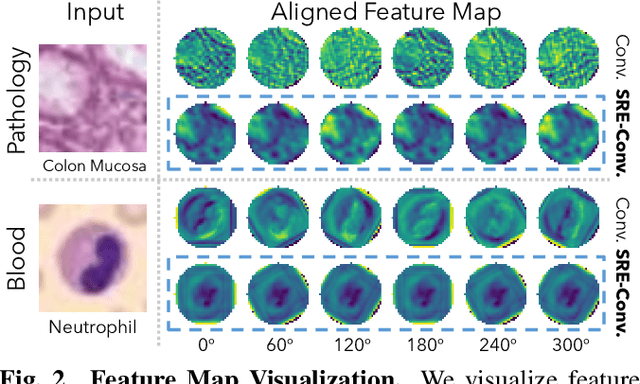
Abstract:Convolutional neural networks (CNNs) are essential tools for computer vision tasks, but they lack traditionally desired properties of extracted features that could further improve model performance, e.g., rotational equivariance. Such properties are ubiquitous in biomedical images, which often lack explicit orientation. While current work largely relies on data augmentation or explicit modules to capture orientation information, this comes at the expense of increased training costs or ineffective approximations of the desired equivariance. To overcome these challenges, we propose a novel and efficient implementation of the Symmetric Rotation-Equivariant (SRE) Convolution (SRE-Conv) kernel, designed to learn rotation-invariant features while simultaneously compressing the model size. The SRE-Conv kernel can easily be incorporated into any CNN backbone. We validate the ability of a deep SRE-CNN to capture equivariance to rotation using the public MedMNISTv2 dataset (16 total tasks). SRE-Conv-CNN demonstrated improved rotated image classification performance accuracy on all 16 test datasets in both 2D and 3D images, all while increasing efficiency with fewer parameters and reduced memory footprint. The code is available at https://github.com/XYPB/SRE-Conv.
Head Motion Degrades Machine Learning Classification of Alzheimer's Disease from Positron Emission Tomography
Jan 14, 2025



Abstract:Brain positron emission tomography (PET) imaging is broadly used in research and clinical routines to study, diagnose, and stage Alzheimer's disease (AD). However, its potential cannot be fully exploited yet due to the lack of portable motion correction solutions, especially in clinical settings. Head motion during data acquisition has indeed been shown to degrade image quality and induces tracer uptake quantification error. In this study, we demonstrate that it also biases machine learning-based AD classification. We start by proposing a binary classification algorithm solely based on PET images. We find that it reaches a high accuracy in classifying motion corrected images into cognitive normal or AD. We demonstrate that the classification accuracy substantially decreases when images lack motion correction, thereby limiting the algorithm's effectiveness and biasing image interpretation. We validate these findings in cohorts of 128 $^{11}$C-UCB-J and 173 $^{18}$F-FDG scans, two tracers highly relevant to the study of AD. Classification accuracies decreased by 10% and 5% on 20 $^{18}$F-FDG and 20 $^{11}$C-UCB-J testing cases, respectively. Our findings underscore the critical need for efficient motion correction methods to make the most of the diagnostic capabilities of PET-based machine learning.
Rate-In: Information-Driven Adaptive Dropout Rates for Improved Inference-Time Uncertainty Estimation
Dec 10, 2024



Abstract:Accurate uncertainty estimation is crucial for deploying neural networks in risk-sensitive applications such as medical diagnosis. Monte Carlo Dropout is a widely used technique for approximating predictive uncertainty by performing stochastic forward passes with dropout during inference. However, using static dropout rates across all layers and inputs can lead to suboptimal uncertainty estimates, as it fails to adapt to the varying characteristics of individual inputs and network layers. Existing approaches optimize dropout rates during training using labeled data, resulting in fixed inference-time parameters that cannot adjust to new data distributions, compromising uncertainty estimates in Monte Carlo simulations. In this paper, we propose Rate-In, an algorithm that dynamically adjusts dropout rates during inference by quantifying the information loss induced by dropout in each layer's feature maps. By treating dropout as controlled noise injection and leveraging information-theoretic principles, Rate-In adapts dropout rates per layer and per input instance without requiring ground truth labels. By quantifying the functional information loss in feature maps, we adaptively tune dropout rates to maintain perceptual quality across diverse medical imaging tasks and architectural configurations. Our extensive empirical study on synthetic data and real-world medical imaging tasks demonstrates that Rate-In improves calibration and sharpens uncertainty estimates compared to fixed or heuristic dropout rates without compromising predictive performance. Rate-In offers a practical, unsupervised, inference-time approach to optimizing dropout for more reliable predictive uncertainty estimation in critical applications.
A Flow-based Truncated Denoising Diffusion Model for Super-resolution Magnetic Resonance Spectroscopic Imaging
Oct 25, 2024Abstract:Magnetic Resonance Spectroscopic Imaging (MRSI) is a non-invasive imaging technique for studying metabolism and has become a crucial tool for understanding neurological diseases, cancers and diabetes. High spatial resolution MRSI is needed to characterize lesions, but in practice MRSI is acquired at low resolution due to time and sensitivity restrictions caused by the low metabolite concentrations. Therefore, there is an imperative need for a post-processing approach to generate high-resolution MRSI from low-resolution data that can be acquired fast and with high sensitivity. Deep learning-based super-resolution methods provided promising results for improving the spatial resolution of MRSI, but they still have limited capability to generate accurate and high-quality images. Recently, diffusion models have demonstrated superior learning capability than other generative models in various tasks, but sampling from diffusion models requires iterating through a large number of diffusion steps, which is time-consuming. This work introduces a Flow-based Truncated Denoising Diffusion Model (FTDDM) for super-resolution MRSI, which shortens the diffusion process by truncating the diffusion chain, and the truncated steps are estimated using a normalizing flow-based network. The network is conditioned on upscaling factors to enable multi-scale super-resolution. To train and evaluate the deep learning models, we developed a 1H-MRSI dataset acquired from 25 high-grade glioma patients. We demonstrate that FTDDM outperforms existing generative models while speeding up the sampling process by over 9-fold compared to the baseline diffusion model. Neuroradiologists' evaluations confirmed the clinical advantages of our method, which also supports uncertainty estimation and sharpness adjustment, extending its potential clinical applications.
* Accepted by Medical Image Analysis (MedIA)
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge