Takuya Toyonaga
Head Motion Degrades Machine Learning Classification of Alzheimer's Disease from Positron Emission Tomography
Jan 14, 2025



Abstract:Brain positron emission tomography (PET) imaging is broadly used in research and clinical routines to study, diagnose, and stage Alzheimer's disease (AD). However, its potential cannot be fully exploited yet due to the lack of portable motion correction solutions, especially in clinical settings. Head motion during data acquisition has indeed been shown to degrade image quality and induces tracer uptake quantification error. In this study, we demonstrate that it also biases machine learning-based AD classification. We start by proposing a binary classification algorithm solely based on PET images. We find that it reaches a high accuracy in classifying motion corrected images into cognitive normal or AD. We demonstrate that the classification accuracy substantially decreases when images lack motion correction, thereby limiting the algorithm's effectiveness and biasing image interpretation. We validate these findings in cohorts of 128 $^{11}$C-UCB-J and 173 $^{18}$F-FDG scans, two tracers highly relevant to the study of AD. Classification accuracies decreased by 10% and 5% on 20 $^{18}$F-FDG and 20 $^{11}$C-UCB-J testing cases, respectively. Our findings underscore the critical need for efficient motion correction methods to make the most of the diagnostic capabilities of PET-based machine learning.
POUR-Net: A Population-Prior-Aided Over-Under-Representation Network for Low-Count PET Attenuation Map Generation
Jan 25, 2024



Abstract:Low-dose PET offers a valuable means of minimizing radiation exposure in PET imaging. However, the prevalent practice of employing additional CT scans for generating attenuation maps (u-map) for PET attenuation correction significantly elevates radiation doses. To address this concern and further mitigate radiation exposure in low-dose PET exams, we propose POUR-Net - an innovative population-prior-aided over-under-representation network that aims for high-quality attenuation map generation from low-dose PET. First, POUR-Net incorporates an over-under-representation network (OUR-Net) to facilitate efficient feature extraction, encompassing both low-resolution abstracted and fine-detail features, for assisting deep generation on the full-resolution level. Second, complementing OUR-Net, a population prior generation machine (PPGM) utilizing a comprehensive CT-derived u-map dataset, provides additional prior information to aid OUR-Net generation. The integration of OUR-Net and PPGM within a cascade framework enables iterative refinement of $\mu$-map generation, resulting in the production of high-quality $\mu$-maps. Experimental results underscore the effectiveness of POUR-Net, showing it as a promising solution for accurate CT-free low-count PET attenuation correction, which also surpasses the performance of previous baseline methods.
A Novel Loss Function Incorporating Imaging Acquisition Physics for PET Attenuation Map Generation using Deep Learning
Sep 03, 2019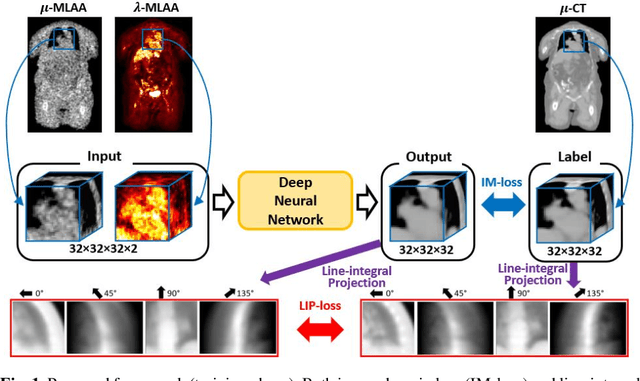
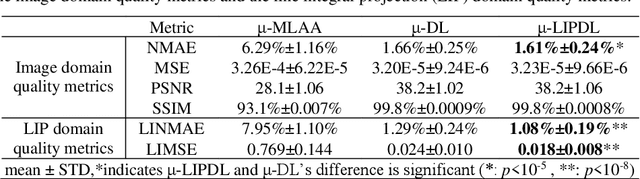
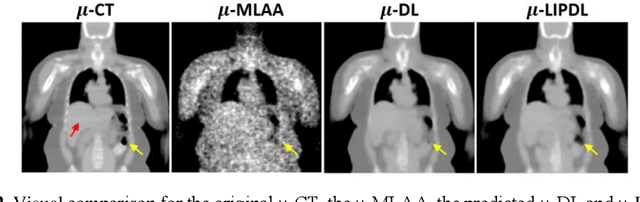

Abstract:In PET/CT imaging, CT is used for PET attenuation correction (AC). Mismatch between CT and PET due to patient body motion results in AC artifacts. In addition, artifact caused by metal, beam-hardening and count-starving in CT itself also introduces inaccurate AC for PET. Maximum likelihood reconstruction of activity and attenuation (MLAA) was proposed to solve those issues by simultaneously reconstructing tracer activity ($\lambda$-MLAA) and attenuation map ($\mu$-MLAA) based on the PET raw data only. However, $\mu$-MLAA suffers from high noise and $\lambda$-MLAA suffers from large bias as compared to the reconstruction using the CT-based attenuation map ($\mu$-CT). Recently, a convolutional neural network (CNN) was applied to predict the CT attenuation map ($\mu$-CNN) from $\lambda$-MLAA and $\mu$-MLAA, in which an image-domain loss (IM-loss) function between the $\mu$-CNN and the ground truth $\mu$-CT was used. However, IM-loss does not directly measure the AC errors according to the PET attenuation physics, where the line-integral projection of the attenuation map ($\mu$) along the path of the two annihilation events, instead of the $\mu$ itself, is used for AC. Therefore, a network trained with the IM-loss may yield suboptimal performance in the $\mu$ generation. Here, we propose a novel line-integral projection loss (LIP-loss) function that incorporates the PET attenuation physics for $\mu$ generation. Eighty training and twenty testing datasets of whole-body 18F-FDG PET and paired ground truth $\mu$-CT were used. Quantitative evaluations showed that the model trained with the additional LIP-loss was able to significantly outperform the model trained solely based on the IM-loss function.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge