Jae Ho Sohn
PatientSim: A Persona-Driven Simulator for Realistic Doctor-Patient Interactions
May 23, 2025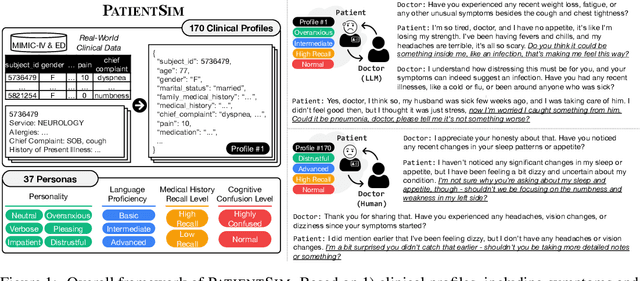
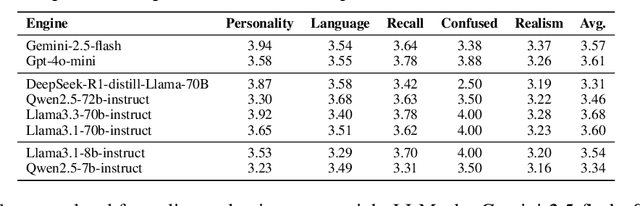
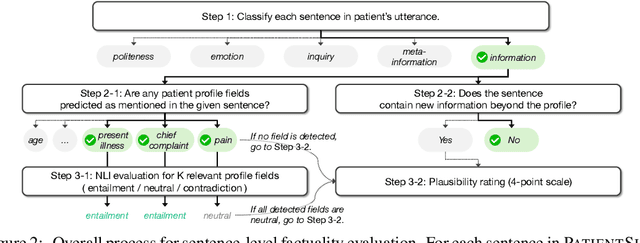
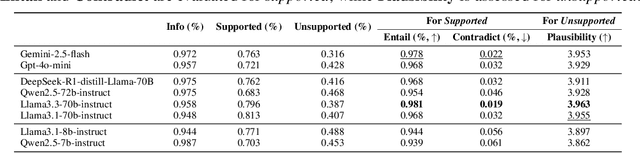
Abstract:Doctor-patient consultations require multi-turn, context-aware communication tailored to diverse patient personas. Training or evaluating doctor LLMs in such settings requires realistic patient interaction systems. However, existing simulators often fail to reflect the full range of personas seen in clinical practice. To address this, we introduce PatientSim, a patient simulator that generates realistic and diverse patient personas for clinical scenarios, grounded in medical expertise. PatientSim operates using: 1) clinical profiles, including symptoms and medical history, derived from real-world data in the MIMIC-ED and MIMIC-IV datasets, and 2) personas defined by four axes: personality, language proficiency, medical history recall level, and cognitive confusion level, resulting in 37 unique combinations. We evaluated eight LLMs for factual accuracy and persona consistency. The top-performing open-source model, Llama 3.3, was validated by four clinicians to confirm the robustness of our framework. As an open-source, customizable platform, PatientSim provides a reproducible and scalable solution that can be customized for specific training needs. Offering a privacy-compliant environment, it serves as a robust testbed for evaluating medical dialogue systems across diverse patient presentations and shows promise as an educational tool for healthcare.
Are LLM-generated plain language summaries truly understandable? A large-scale crowdsourced evaluation
May 15, 2025Abstract:Plain language summaries (PLSs) are essential for facilitating effective communication between clinicians and patients by making complex medical information easier for laypeople to understand and act upon. Large language models (LLMs) have recently shown promise in automating PLS generation, but their effectiveness in supporting health information comprehension remains unclear. Prior evaluations have generally relied on automated scores that do not measure understandability directly, or subjective Likert-scale ratings from convenience samples with limited generalizability. To address these gaps, we conducted a large-scale crowdsourced evaluation of LLM-generated PLSs using Amazon Mechanical Turk with 150 participants. We assessed PLS quality through subjective Likert-scale ratings focusing on simplicity, informativeness, coherence, and faithfulness; and objective multiple-choice comprehension and recall measures of reader understanding. Additionally, we examined the alignment between 10 automated evaluation metrics and human judgments. Our findings indicate that while LLMs can generate PLSs that appear indistinguishable from human-written ones in subjective evaluations, human-written PLSs lead to significantly better comprehension. Furthermore, automated evaluation metrics fail to reflect human judgment, calling into question their suitability for evaluating PLSs. This is the first study to systematically evaluate LLM-generated PLSs based on both reader preferences and comprehension outcomes. Our findings highlight the need for evaluation frameworks that move beyond surface-level quality and for generation methods that explicitly optimize for layperson comprehension.
Are Generative AI systems Capable of Supporting Information Needs of Patients?
Jan 31, 2024Abstract:Patients managing a complex illness such as cancer face a complex information challenge where they not only must learn about their illness but also how to manage it. Close interaction with healthcare experts (radiologists, oncologists) can improve patient learning and thereby, their disease outcome. However, this approach is resource intensive and takes expert time away from other critical tasks. Given the recent advancements in Generative AI models aimed at improving the healthcare system, our work investigates whether and how generative visual question answering systems can responsibly support patient information needs in the context of radiology imaging data. We conducted a formative need-finding study in which participants discussed chest computed tomography (CT) scans and associated radiology reports of a fictitious close relative with a cardiothoracic radiologist. Using thematic analysis of the conversation between participants and medical experts, we identified commonly occurring themes across interactions, including clarifying medical terminology, locating the problems mentioned in the report in the scanned image, understanding disease prognosis, discussing the next diagnostic steps, and comparing treatment options. Based on these themes, we evaluated two state-of-the-art generative visual language models against the radiologist's responses. Our results reveal variability in the quality of responses generated by the models across various themes. We highlight the importance of patient-facing generative AI systems to accommodate a diverse range of conversational themes, catering to the real-world informational needs of patients.
Conformal Language Modeling
Jun 16, 2023Abstract:We propose a novel approach to conformal prediction for generative language models (LMs). Standard conformal prediction produces prediction sets -- in place of single predictions -- that have rigorous, statistical performance guarantees. LM responses are typically sampled from the model's predicted distribution over the large, combinatorial output space of natural language. Translating this process to conformal prediction, we calibrate a stopping rule for sampling different outputs from the LM that get added to a growing set of candidates until we are confident that the output set is sufficient. Since some samples may be low-quality, we also simultaneously calibrate and apply a rejection rule for removing candidates from the output set to reduce noise. Similar to conformal prediction, we prove that the sampled set returned by our procedure contains at least one acceptable answer with high probability, while still being empirically precise (i.e., small) on average. Furthermore, within this set of candidate responses, we show that we can also accurately identify subsets of individual components -- such as phrases or sentences -- that are each independently correct (e.g., that are not "hallucinations"), again with statistical guarantees. We demonstrate the promise of our approach on multiple tasks in open-domain question answering, text summarization, and radiology report generation using different LM variants.
Automatic Hip Fracture Identification and Functional Subclassification with Deep Learning
Sep 10, 2019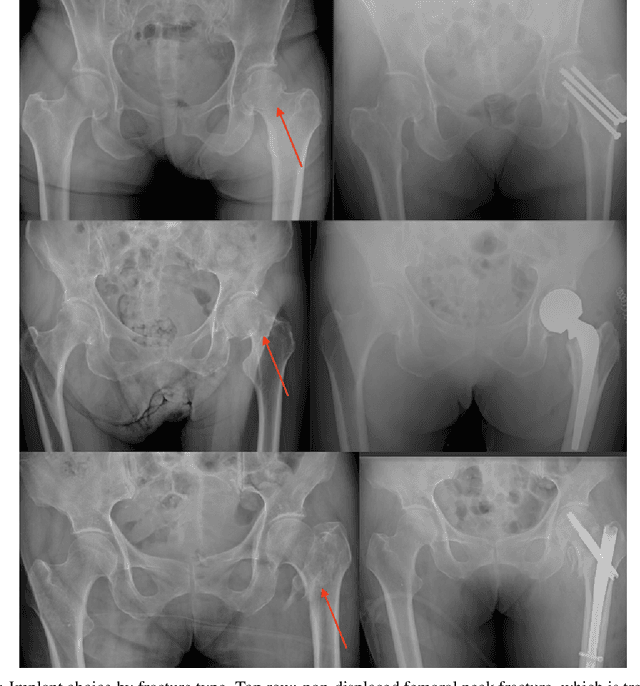
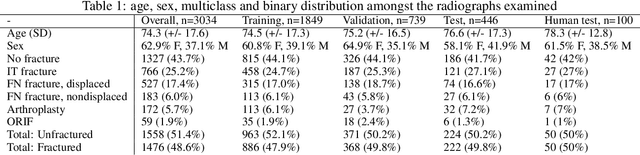
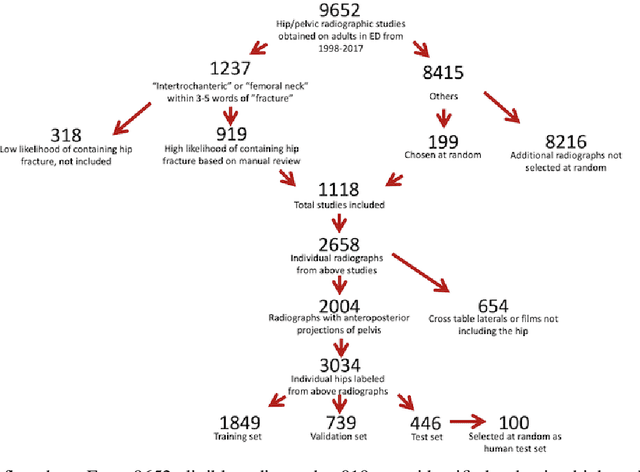
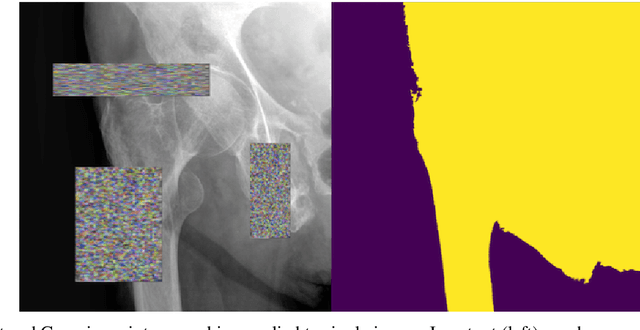
Abstract:Purpose: Hip fractures are a common cause of morbidity and mortality. Automatic identification and classification of hip fractures using deep learning may improve outcomes by reducing diagnostic errors and decreasing time to operation. Methods: Hip and pelvic radiographs from 1118 studies were reviewed and 3034 hips were labeled via bounding boxes and classified as normal, displaced femoral neck fracture, nondisplaced femoral neck fracture, intertrochanteric fracture, previous ORIF, or previous arthroplasty. A deep learning-based object detection model was trained to automate the placement of the bounding boxes. A Densely Connected Convolutional Neural Network (DenseNet) was trained on a subset of the bounding box images, and its performance evaluated on a held out test set and by comparison on a 100-image subset to two groups of human observers: fellowship-trained radiologists and orthopaedists, and senior residents in emergency medicine, radiology, and orthopaedics. Results: The binary accuracy for fracture of our model was 93.8% (95% CI, 91.3-95.8%), with sensitivity of 92.7% (95% CI, 88.7-95.6%), and specificity 95.0% (95% CI, 91.5-97.3%). Multiclass classification accuracy was 90.4% (95% CI, 87.4-92.9%). When compared to human observers, our model achieved at least expert-level classification under all conditions. Additionally, when the model was used as an aid, human performance improved, with aided resident performance approximating unaided fellowship-trained expert performance. Conclusions: Our deep learning model identified and classified hip fractures with at least expert-level accuracy, and when used as an aid improved human performance, with aided resident performance approximating that of unaided fellowship-trained attendings.
Joint Correction of Attenuation and Scatter Using Deep Convolutional Neural Networks (DCNN) for Time-of-Flight PET
Nov 28, 2018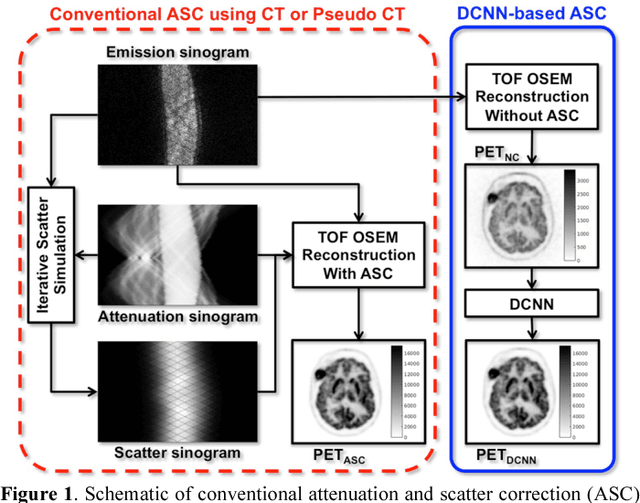
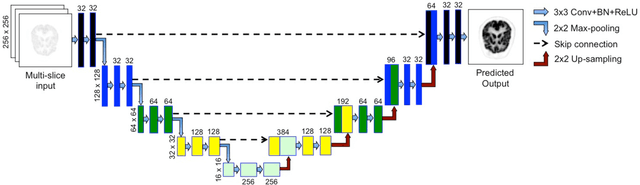
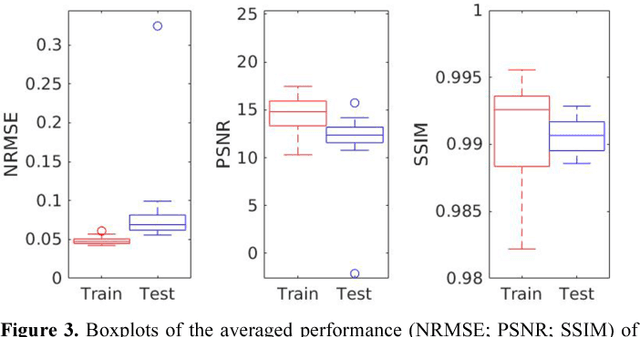
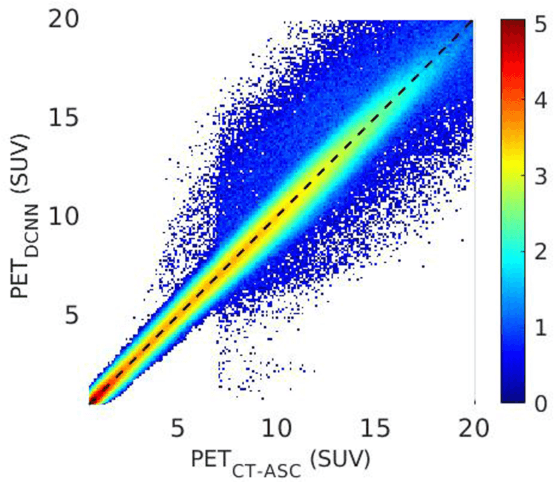
Abstract:Deep convolutional neural networks (DCNN) have demonstrated its capability to convert MR image to pseudo CT for PET attenuation correction in PET/MRI. Conventionally, attenuated events are corrected in sinogram space using attenuation maps derived from CT or MR-derived pseudo CT. Separately, scattered events are iteratively estimated by a 3D model-based simulation using down-sampled attenuation and emission sinograms. However, no studies have investigated joint correction of attenuation and scatter using DCNN in image space. Therefore, we aim to develop and optimize a DCNN model for attenuation and scatter correction (ASC) simultaneously in PET image space without additional anatomical imaging or time-consuming iterative scatter simulation. For the first time, we demonstrated the feasibility of directly producing PET images corrected for attenuation and scatter using DCNN (PET-DCNN) from noncorrected PET (PET-NC) images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge