Carol C. Wu
Interpreting Radiologist's Intention from Eye Movements in Chest X-ray Diagnosis
Jul 16, 2025Abstract:Radiologists rely on eye movements to navigate and interpret medical images. A trained radiologist possesses knowledge about the potential diseases that may be present in the images and, when searching, follows a mental checklist to locate them using their gaze. This is a key observation, yet existing models fail to capture the underlying intent behind each fixation. In this paper, we introduce a deep learning-based approach, RadGazeIntent, designed to model this behavior: having an intention to find something and actively searching for it. Our transformer-based architecture processes both the temporal and spatial dimensions of gaze data, transforming fine-grained fixation features into coarse, meaningful representations of diagnostic intent to interpret radiologists' goals. To capture the nuances of radiologists' varied intention-driven behaviors, we process existing medical eye-tracking datasets to create three intention-labeled subsets: RadSeq (Systematic Sequential Search), RadExplore (Uncertainty-driven Exploration), and RadHybrid (Hybrid Pattern). Experimental results demonstrate RadGazeIntent's ability to predict which findings radiologists are examining at specific moments, outperforming baseline methods across all intention-labeled datasets.
Beyond the First Read: AI-Assisted Perceptual Error Detection in Chest Radiography Accounting for Interobserver Variability
Jun 16, 2025Abstract:Chest radiography is widely used in diagnostic imaging. However, perceptual errors -- especially overlooked but visible abnormalities -- remain common and clinically significant. Current workflows and AI systems provide limited support for detecting such errors after interpretation and often lack meaningful human--AI collaboration. We introduce RADAR (Radiologist--AI Diagnostic Assistance and Review), a post-interpretation companion system. RADAR ingests finalized radiologist annotations and CXR images, then performs regional-level analysis to detect and refer potentially missed abnormal regions. The system supports a "second-look" workflow and offers suggested regions of interest (ROIs) rather than fixed labels to accommodate inter-observer variation. We evaluated RADAR on a simulated perceptual-error dataset derived from de-identified CXR cases, using F1 score and Intersection over Union (IoU) as primary metrics. RADAR achieved a recall of 0.78, precision of 0.44, and an F1 score of 0.56 in detecting missed abnormalities in the simulated perceptual-error dataset. Although precision is moderate, this reduces over-reliance on AI by encouraging radiologist oversight in human--AI collaboration. The median IoU was 0.78, with more than 90% of referrals exceeding 0.5 IoU, indicating accurate regional localization. RADAR effectively complements radiologist judgment, providing valuable post-read support for perceptual-error detection in CXR interpretation. Its flexible ROI suggestions and non-intrusive integration position it as a promising tool in real-world radiology workflows. To facilitate reproducibility and further evaluation, we release a fully open-source web implementation alongside a simulated error dataset. All code, data, demonstration videos, and the application are publicly available at https://github.com/avutukuri01/RADAR.
FG-CXR: A Radiologist-Aligned Gaze Dataset for Enhancing Interpretability in Chest X-Ray Report Generation
Nov 23, 2024



Abstract:Developing an interpretable system for generating reports in chest X-ray (CXR) analysis is becoming increasingly crucial in Computer-aided Diagnosis (CAD) systems, enabling radiologists to comprehend the decisions made by these systems. Despite the growth of diverse datasets and methods focusing on report generation, there remains a notable gap in how closely these models' generated reports align with the interpretations of real radiologists. In this study, we tackle this challenge by initially introducing Fine-Grained CXR (FG-CXR) dataset, which provides fine-grained paired information between the captions generated by radiologists and the corresponding gaze attention heatmaps for each anatomy. Unlike existing datasets that include a raw sequence of gaze alongside a report, with significant misalignment between gaze location and report content, our FG-CXR dataset offers a more grained alignment between gaze attention and diagnosis transcript. Furthermore, our analysis reveals that simply applying black-box image captioning methods to generate reports cannot adequately explain which information in CXR is utilized and how long needs to attend to accurately generate reports. Consequently, we propose a novel explainable radiologist's attention generator network (Gen-XAI) that mimics the diagnosis process of radiologists, explicitly constraining its output to closely align with both radiologist's gaze attention and transcript. Finally, we perform extensive experiments to illustrate the effectiveness of our method. Our datasets and checkpoint is available at https://github.com/UARK-AICV/FG-CXR.
GazeSearch: Radiology Findings Search Benchmark
Nov 08, 2024



Abstract:Medical eye-tracking data is an important information source for understanding how radiologists visually interpret medical images. This information not only improves the accuracy of deep learning models for X-ray analysis but also their interpretability, enhancing transparency in decision-making. However, the current eye-tracking data is dispersed, unprocessed, and ambiguous, making it difficult to derive meaningful insights. Therefore, there is a need to create a new dataset with more focus and purposeful eyetracking data, improving its utility for diagnostic applications. In this work, we propose a refinement method inspired by the target-present visual search challenge: there is a specific finding and fixations are guided to locate it. After refining the existing eye-tracking datasets, we transform them into a curated visual search dataset, called GazeSearch, specifically for radiology findings, where each fixation sequence is purposefully aligned to the task of locating a particular finding. Subsequently, we introduce a scan path prediction baseline, called ChestSearch, specifically tailored to GazeSearch. Finally, we employ the newly introduced GazeSearch as a benchmark to evaluate the performance of current state-of-the-art methods, offering a comprehensive assessment for visual search in the medical imaging domain.
Multimodal Learning and Cognitive Processes in Radiology: MedGaze for Chest X-ray Scanpath Prediction
Jun 28, 2024



Abstract:Predicting human gaze behavior within computer vision is integral for developing interactive systems that can anticipate user attention, address fundamental questions in cognitive science, and hold implications for fields like human-computer interaction (HCI) and augmented/virtual reality (AR/VR) systems. Despite methodologies introduced for modeling human eye gaze behavior, applying these models to medical imaging for scanpath prediction remains unexplored. Our proposed system aims to predict eye gaze sequences from radiology reports and CXR images, potentially streamlining data collection and enhancing AI systems using larger datasets. However, predicting human scanpaths on medical images presents unique challenges due to the diverse nature of abnormal regions. Our model predicts fixation coordinates and durations critical for medical scanpath prediction, outperforming existing models in the computer vision community. Utilizing a two-stage training process and large publicly available datasets, our approach generates static heatmaps and eye gaze videos aligned with radiology reports, facilitating comprehensive analysis. We validate our approach by comparing its performance with state-of-the-art methods and assessing its generalizability among different radiologists, introducing novel strategies to model radiologists' search patterns during CXR image diagnosis. Based on the radiologist's evaluation, MedGaze can generate human-like gaze sequences with a high focus on relevant regions over the CXR images. It sometimes also outperforms humans in terms of redundancy and randomness in the scanpaths.
Enhancing Radiological Diagnosis: A Collaborative Approach Integrating AI and Human Expertise for Visual Miss Correction
Jun 28, 2024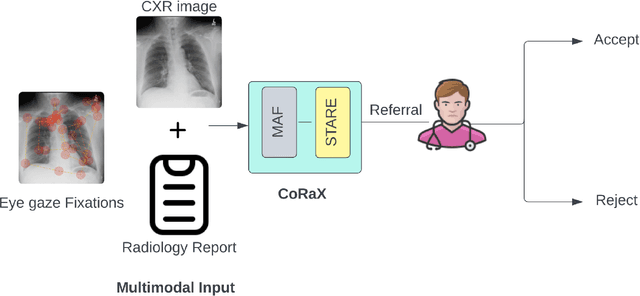

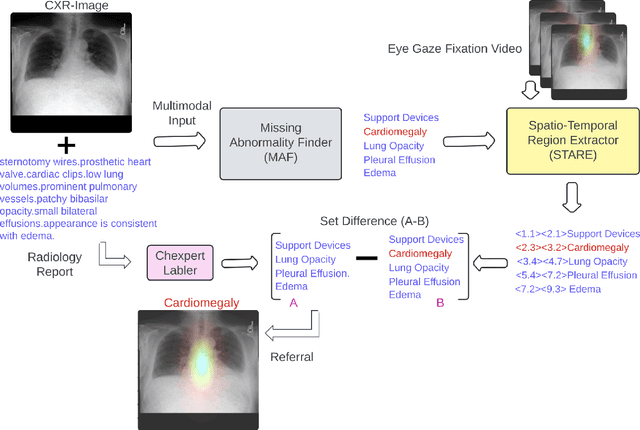

Abstract:Human-AI collaboration to identify and correct perceptual errors in chest radiographs has not been previously explored. This study aimed to develop a collaborative AI system, CoRaX, which integrates eye gaze data and radiology reports to enhance diagnostic accuracy in chest radiology by pinpointing perceptual errors and refining the decision-making process. Using public datasets REFLACX and EGD-CXR, the study retrospectively developed CoRaX, employing a large multimodal model to analyze image embeddings, eye gaze data, and radiology reports. The system's effectiveness was evaluated based on its referral-making process, the quality of referrals, and performance in collaborative diagnostic settings. CoRaX was tested on a simulated error dataset of 271 samples with 28% (93 of 332) missed abnormalities. The system corrected 21% (71 of 332) of these errors, leaving 7% (22 of 312) unresolved. The Referral-Usefulness score, indicating the accuracy of predicted regions for all true referrals, was 0.63 (95% CI 0.59, 0.68). The Total-Usefulness score, reflecting the diagnostic accuracy of CoRaX's interactions with radiologists, showed that 84% (237 of 280) of these interactions had a score above 0.40. In conclusion, CoRaX efficiently collaborates with radiologists to address perceptual errors across various abnormalities, with potential applications in the education and training of novice radiologists.
Radiologist-Level COVID-19 Detection Using CT Scans with Detail-Oriented Capsule Networks
Apr 16, 2020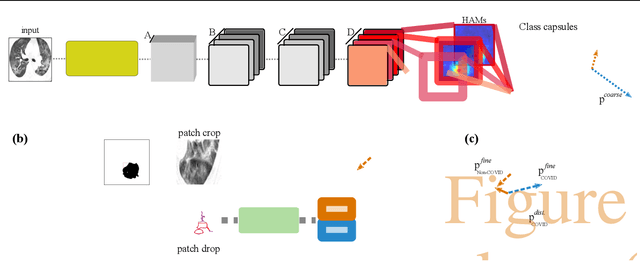
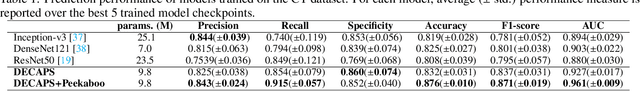
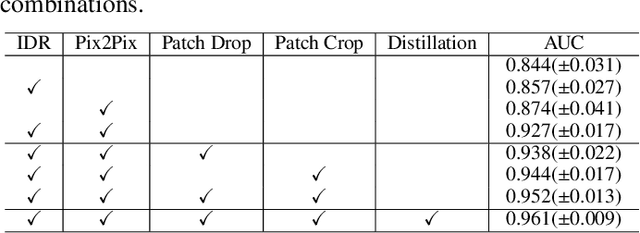
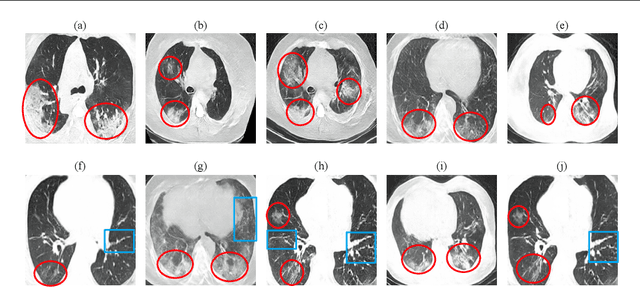
Abstract:Radiographic images offer an alternative method for the rapid screening and monitoring of Coronavirus Disease 2019 (COVID-19) patients. This approach is limited by the shortage of radiology experts who can provide a timely interpretation of these images. Motivated by this challenge, our paper proposes a novel learning architecture, called Detail-Oriented Capsule Networks (DECAPS), for the automatic diagnosis of COVID-19 from Computed Tomography (CT) scans. Our network combines the strength of Capsule Networks with several architecture improvements meant to boost classification accuracies. First, DECAPS uses an Inverted Dynamic Routing mechanism which increases model stability by preventing the passage of information from non-descriptive regions. Second, DECAPS employs a Peekaboo training procedure which uses a two-stage patch crop and drop strategy to encourage the network to generate activation maps for every target concept. The network then uses the activation maps to focus on regions of interest and combines both coarse and fine-grained representations of the data. Finally, we use a data augmentation method based on conditional generative adversarial networks to deal with the issue of data scarcity. Our model achieves 84.3% precision, 91.5% recall, and 96.1% area under the ROC curve, significantly outperforming state-of-the-art methods. We compare the performance of the DECAPS model with three experienced, well-trained thoracic radiologists and show that the architecture significantly outperforms them. While further studies on larger datasets are required to confirm this finding, our results imply that architectures like DECAPS can be used to assist radiologists in the CT scan mediated diagnosis of COVID-19.
DropConnect Is Effective in Modeling Uncertainty of Bayesian Deep Networks
Jun 07, 2019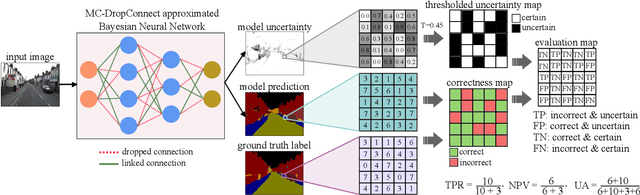
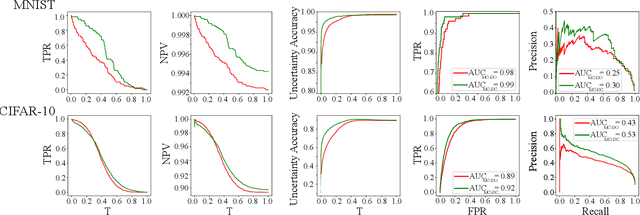
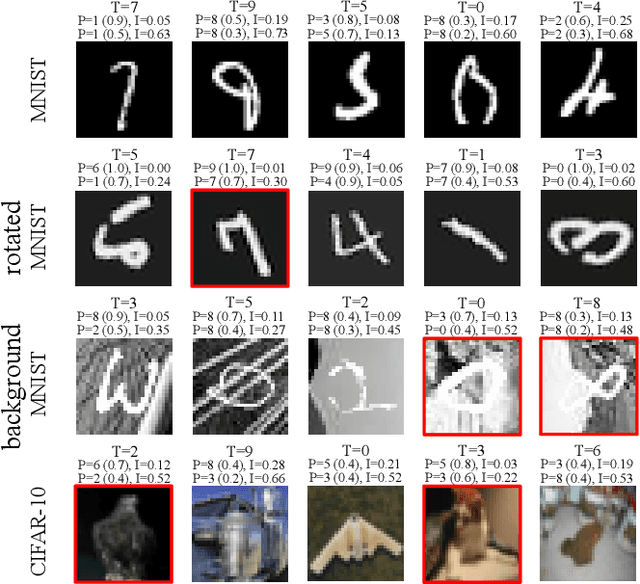
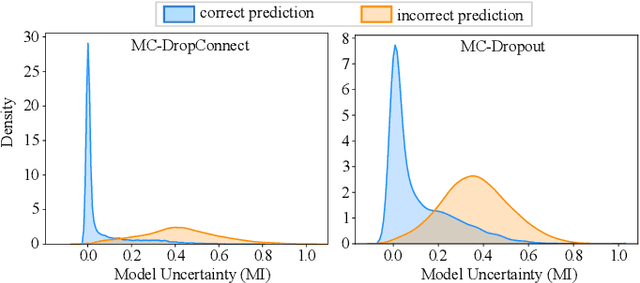
Abstract:Deep neural networks (DNNs) have achieved state-of-the-art performances in many important domains, including medical diagnosis, security, and autonomous driving. In these domains where safety is highly critical, an erroneous decision can result in serious consequences. While a perfect prediction accuracy is not always achievable, recent work on Bayesian deep networks shows that it is possible to know when DNNs are more likely to make mistakes. Knowing what DNNs do not know is desirable to increase the safety of deep learning technology in sensitive applications. Bayesian neural networks attempt to address this challenge. However, traditional approaches are computationally intractable and do not scale well to large, complex neural network architectures. In this paper, we develop a theoretical framework to approximate Bayesian inference for DNNs by imposing a Bernoulli distribution on the model weights. This method, called MC-DropConnect, gives us a tool to represent the model uncertainty with little change in the overall model structure or computational cost. We extensively validate the proposed algorithm on multiple network architectures and datasets for classification and semantic segmentation tasks. We also propose new metrics to quantify the uncertainty estimates. This enables an objective comparison between MC-DropConnect and prior approaches. Our empirical results demonstrate that the proposed framework yields significant improvement in both prediction accuracy and uncertainty estimation quality compared to the state of the art.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge