Candice W. Bolan
Large-Scale Multi-Center CT and MRI Segmentation of Pancreas with Deep Learning
May 20, 2024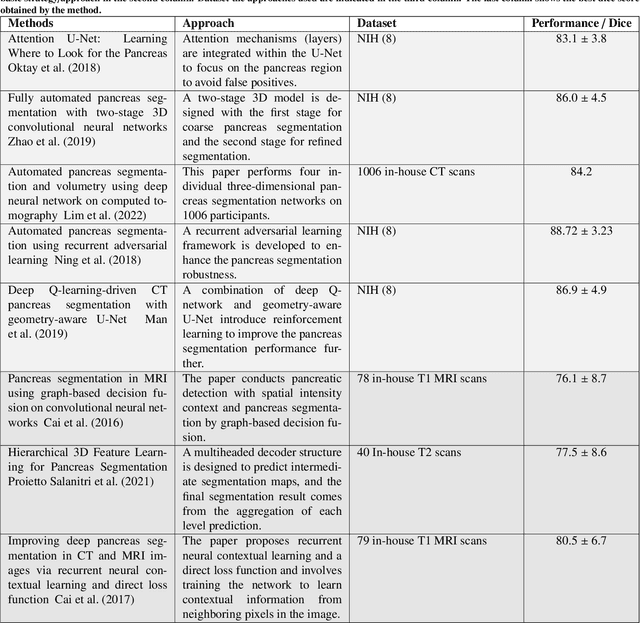
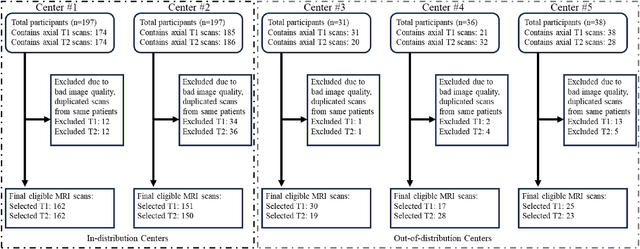
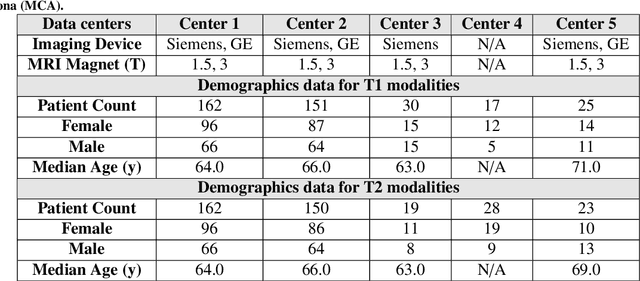
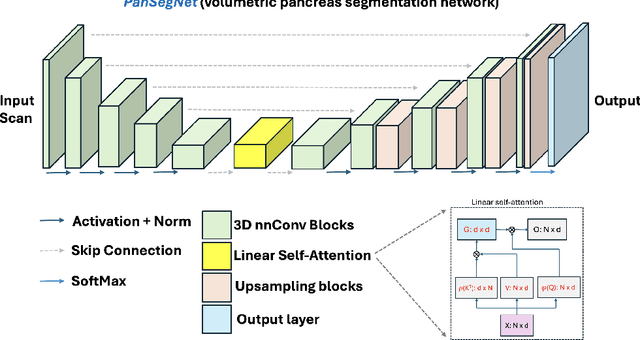
Abstract:Automated volumetric segmentation of the pancreas on cross-sectional imaging is needed for diagnosis and follow-up of pancreatic diseases. While CT-based pancreatic segmentation is more established, MRI-based segmentation methods are understudied, largely due to a lack of publicly available datasets, benchmarking research efforts, and domain-specific deep learning methods. In this retrospective study, we collected a large dataset (767 scans from 499 participants) of T1-weighted (T1W) and T2-weighted (T2W) abdominal MRI series from five centers between March 2004 and November 2022. We also collected CT scans of 1,350 patients from publicly available sources for benchmarking purposes. We developed a new pancreas segmentation method, called PanSegNet, combining the strengths of nnUNet and a Transformer network with a new linear attention module enabling volumetric computation. We tested PanSegNet's accuracy in cross-modality (a total of 2,117 scans) and cross-center settings with Dice and Hausdorff distance (HD95) evaluation metrics. We used Cohen's kappa statistics for intra and inter-rater agreement evaluation and paired t-tests for volume and Dice comparisons, respectively. For segmentation accuracy, we achieved Dice coefficients of 88.3% (std: 7.2%, at case level) with CT, 85.0% (std: 7.9%) with T1W MRI, and 86.3% (std: 6.4%) with T2W MRI. There was a high correlation for pancreas volume prediction with R^2 of 0.91, 0.84, and 0.85 for CT, T1W, and T2W, respectively. We found moderate inter-observer (0.624 and 0.638 for T1W and T2W MRI, respectively) and high intra-observer agreement scores. All MRI data is made available at https://osf.io/kysnj/. Our source code is available at https://github.com/NUBagciLab/PaNSegNet.
Neural Transformers for Intraductal Papillary Mucosal Neoplasms (IPMN) Classification in MRI images
Jun 21, 2022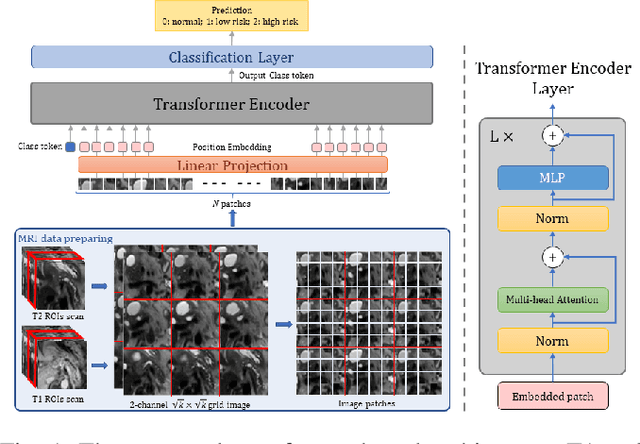
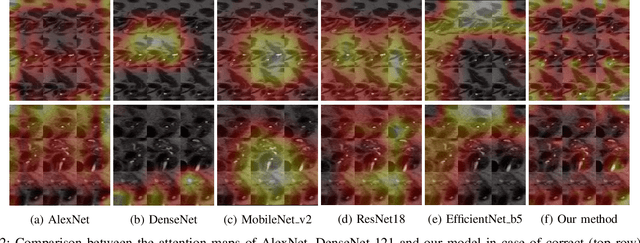

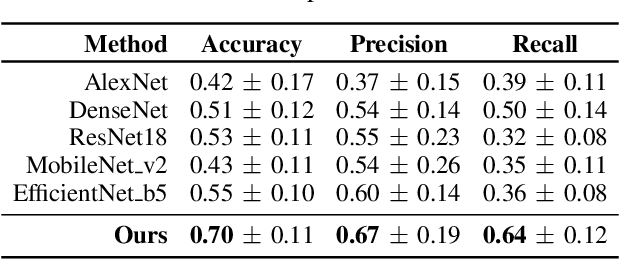
Abstract:Early detection of precancerous cysts or neoplasms, i.e., Intraductal Papillary Mucosal Neoplasms (IPMN), in pancreas is a challenging and complex task, and it may lead to a more favourable outcome. Once detected, grading IPMNs accurately is also necessary, since low-risk IPMNs can be under surveillance program, while high-risk IPMNs have to be surgically resected before they turn into cancer. Current standards (Fukuoka and others) for IPMN classification show significant intra- and inter-operator variability, beside being error-prone, making a proper diagnosis unreliable. The established progress in artificial intelligence, through the deep learning paradigm, may provide a key tool for an effective support to medical decision for pancreatic cancer. In this work, we follow this trend, by proposing a novel AI-based IPMN classifier that leverages the recent success of transformer networks in generalizing across a wide variety of tasks, including vision ones. We specifically show that our transformer-based model exploits pre-training better than standard convolutional neural networks, thus supporting the sought architectural universalism of transformers in vision, including the medical image domain and it allows for a better interpretation of the obtained results.
INN: Inflated Neural Networks for IPMN Diagnosis
Jun 30, 2019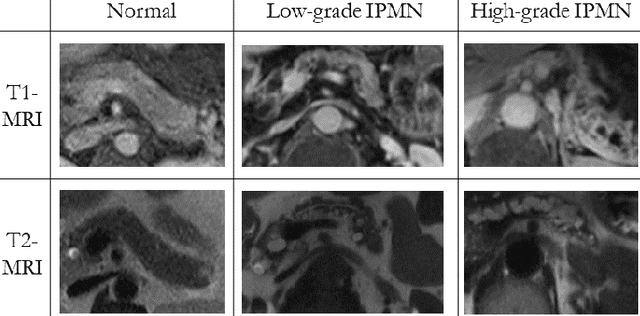
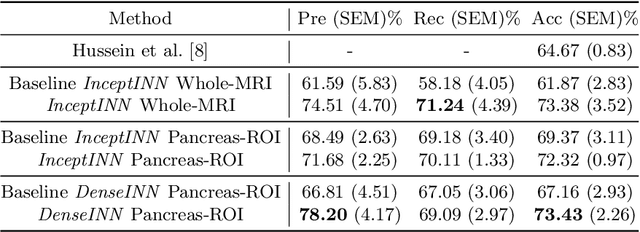
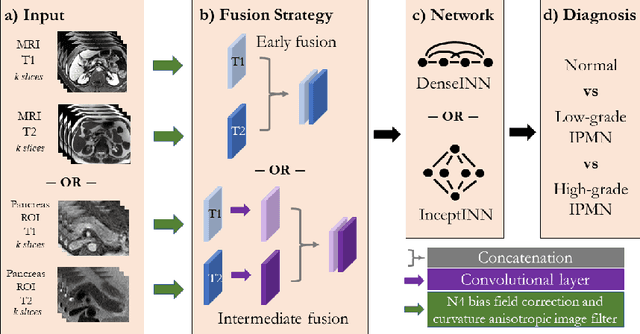

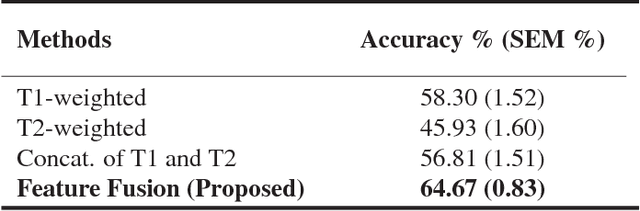
Abstract:Intraductal papillary mucinous neoplasm (IPMN) is a precursor to pancreatic ductal adenocarcinoma. While over half of patients are diagnosed with pancreatic cancer at a distant stage, patients who are diagnosed early enjoy a much higher 5-year survival rate of $34\%$ compared to $3\%$ in the former; hence, early diagnosis is key. Unique challenges in the medical imaging domain such as extremely limited annotated data sets and typically large 3D volumetric data have made it difficult for deep learning to secure a strong foothold. In this work, we construct two novel "inflated" deep network architectures, $\textit{InceptINN}$ and $\textit{DenseINN}$, for the task of diagnosing IPMN from multisequence (T1 and T2) MRI. These networks inflate their 2D layers to 3D and bootstrap weights from their 2D counterparts (Inceptionv3 and DenseNet121 respectively) trained on ImageNet to the new 3D kernels. We also extend the inflation process by further expanding the pre-trained kernels to handle any number of input modalities and different fusion strategies. This is one of the first studies to train an end-to-end deep network on multisequence MRI for IPMN diagnosis, and shows that our proposed novel inflated network architectures are able to handle the extremely limited training data (139 MRI scans), while providing an absolute improvement of $8.76\%$ in accuracy for diagnosing IPMN over the current state-of-the-art. Code is publicly available at https://github.com/lalonderodney/INN-Inflated-Neural-Nets.
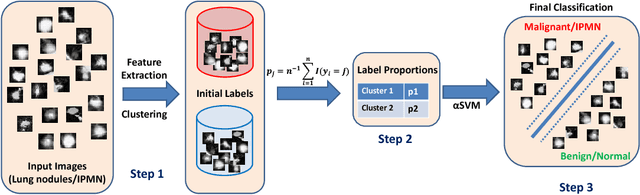
Supervised and Unsupervised Tumor Characterization in the Deep Learning Era
Jul 29, 2018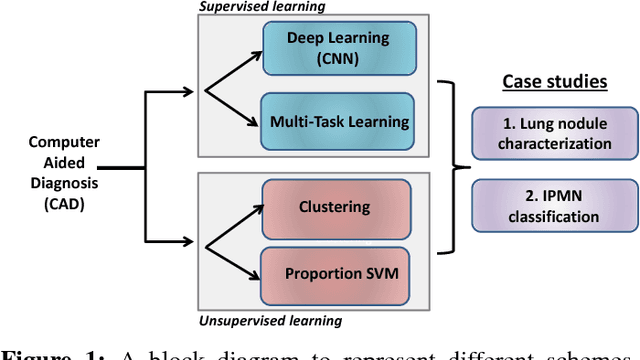

Abstract:Cancer is among the leading causes of death worldwide. Risk stratification of cancer tumors in radiology images can be improved with computer-aided diagnosis (CAD) tools which can be made faster and more accurate. Tumor characterization through CADs can enable non-invasive cancer staging and prognosis, and foster personalized treatment planning as a part of precision medicine. In this study, we propose both supervised and unsupervised machine learning strategies to improve tumor characterization. Our first approach is based on supervised learning for which we demonstrate significant gains in deep learning algorithms, particularly by utilizing a 3D Convolutional Neural Network along with transfer learning. Motivated by the radiologists' interpretations of the scans, we then show how to incorporate task dependent feature representations into a CAD system via a "graph-regularized sparse Multi-Task Learning (MTL)" framework. In the second approach, we explore an unsupervised scheme to address the limited availability of labeled training data, a common problem in medical imaging applications. Inspired by learning from label proportion (LLP) approaches, we propose a new algorithm, proportion-SVM, to characterize tumor types. We also seek the answer to the fundamental question about the goodness of "deep features" for unsupervised tumor classification. We evaluate our proposed approaches (both supervised and unsupervised) on two different tumor diagnosis challenges: lung and pancreas with 1018 CT and 171 MRI scans respectively.
Deep Multi-Modal Classification of Intraductal Papillary Mucinous Neoplasms (IPMN) with Canonical Correlation Analysis
Apr 27, 2018
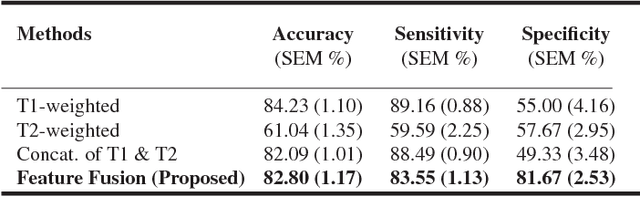
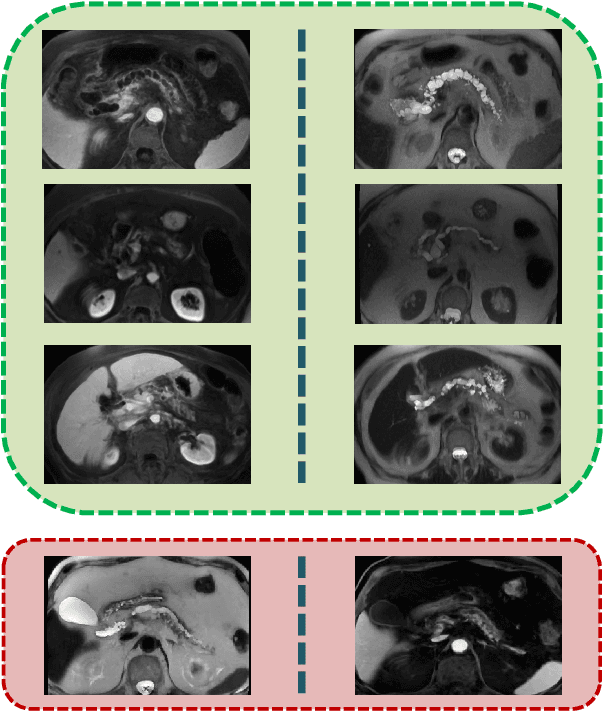
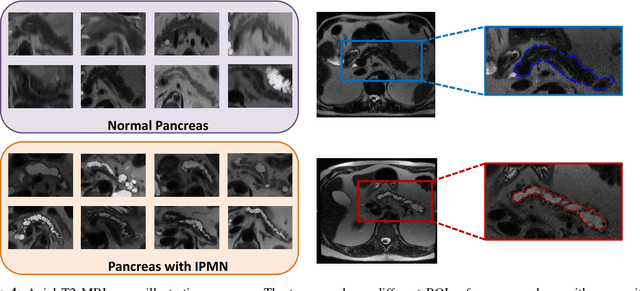
Abstract:Pancreatic cancer has the poorest prognosis among all cancer types. Intraductal Papillary Mucinous Neoplasms (IPMNs) are radiographically identifiable precursors to pancreatic cancer; hence, early detection and precise risk assessment of IPMN are vital. In this work, we propose a Convolutional Neural Network (CNN) based computer aided diagnosis (CAD) system to perform IPMN diagnosis and risk assessment by utilizing multi-modal MRI. In our proposed approach, we use minimum and maximum intensity projections to ease the annotation variations among different slices and type of MRIs. Then, we present a CNN to obtain deep feature representation corresponding to each MRI modality (T1-weighted and T2-weighted). At the final step, we employ canonical correlation analysis (CCA) to perform a fusion operation at the feature level, leading to discriminative canonical correlation features. Extracted features are used for classification. Our results indicate significant improvements over other potential approaches to solve this important problem. The proposed approach doesn't require explicit sample balancing in cases of imbalance between positive and negative examples. To the best of our knowledge, our study is the first to automatically diagnose IPMN using multi-modal MRI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge