Sanne Hoogenboom
Large-Scale Multi-Center CT and MRI Segmentation of Pancreas with Deep Learning
May 20, 2024
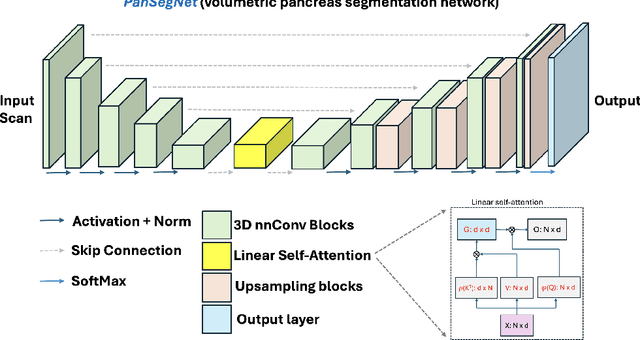
Abstract:Automated volumetric segmentation of the pancreas on cross-sectional imaging is needed for diagnosis and follow-up of pancreatic diseases. While CT-based pancreatic segmentation is more established, MRI-based segmentation methods are understudied, largely due to a lack of publicly available datasets, benchmarking research efforts, and domain-specific deep learning methods. In this retrospective study, we collected a large dataset (767 scans from 499 participants) of T1-weighted (T1W) and T2-weighted (T2W) abdominal MRI series from five centers between March 2004 and November 2022. We also collected CT scans of 1,350 patients from publicly available sources for benchmarking purposes. We developed a new pancreas segmentation method, called PanSegNet, combining the strengths of nnUNet and a Transformer network with a new linear attention module enabling volumetric computation. We tested PanSegNet's accuracy in cross-modality (a total of 2,117 scans) and cross-center settings with Dice and Hausdorff distance (HD95) evaluation metrics. We used Cohen's kappa statistics for intra and inter-rater agreement evaluation and paired t-tests for volume and Dice comparisons, respectively. For segmentation accuracy, we achieved Dice coefficients of 88.3% (std: 7.2%, at case level) with CT, 85.0% (std: 7.9%) with T1W MRI, and 86.3% (std: 6.4%) with T2W MRI. There was a high correlation for pancreas volume prediction with R^2 of 0.91, 0.84, and 0.85 for CT, T1W, and T2W, respectively. We found moderate inter-observer (0.624 and 0.638 for T1W and T2W MRI, respectively) and high intra-observer agreement scores. All MRI data is made available at https://osf.io/kysnj/. Our source code is available at https://github.com/NUBagciLab/PaNSegNet.
A Critical Appraisal of Data Augmentation Methods for Imaging-Based Medical Diagnosis Applications
Dec 14, 2022

Abstract:Current data augmentation techniques and transformations are well suited for improving the size and quality of natural image datasets but are not yet optimized for medical imaging. We hypothesize that sub-optimal data augmentations can easily distort or occlude medical images, leading to false positives or negatives during patient diagnosis, prediction, or therapy/surgery evaluation. In our experimental results, we found that utilizing commonly used intensity-based data augmentation distorts the MRI scans and leads to texture information loss, thus negatively affecting the overall performance of classification. Additionally, we observed that commonly used data augmentation methods cannot be used with a plug-and-play approach in medical imaging, and requires manual tuning and adjustment.
Neural Transformers for Intraductal Papillary Mucosal Neoplasms (IPMN) Classification in MRI images
Jun 21, 2022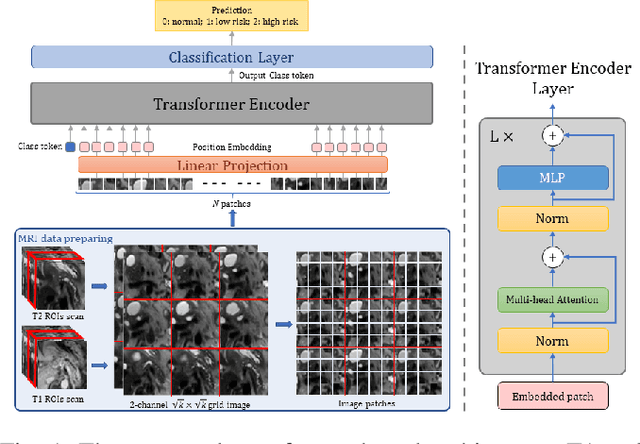
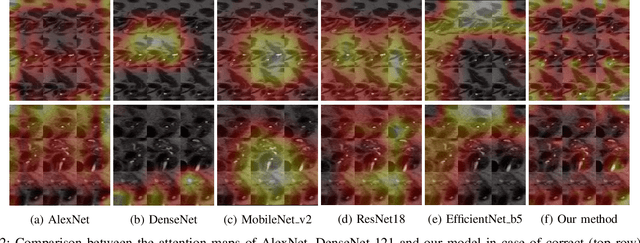

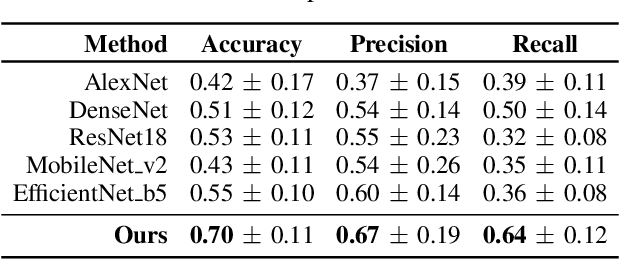
Abstract:Early detection of precancerous cysts or neoplasms, i.e., Intraductal Papillary Mucosal Neoplasms (IPMN), in pancreas is a challenging and complex task, and it may lead to a more favourable outcome. Once detected, grading IPMNs accurately is also necessary, since low-risk IPMNs can be under surveillance program, while high-risk IPMNs have to be surgically resected before they turn into cancer. Current standards (Fukuoka and others) for IPMN classification show significant intra- and inter-operator variability, beside being error-prone, making a proper diagnosis unreliable. The established progress in artificial intelligence, through the deep learning paradigm, may provide a key tool for an effective support to medical decision for pancreatic cancer. In this work, we follow this trend, by proposing a novel AI-based IPMN classifier that leverages the recent success of transformer networks in generalizing across a wide variety of tasks, including vision ones. We specifically show that our transformer-based model exploits pre-training better than standard convolutional neural networks, thus supporting the sought architectural universalism of transformers in vision, including the medical image domain and it allows for a better interpretation of the obtained results.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge