Anant Vemuri
Video-rate multispectral imaging in laparoscopic surgery: First-in-human application
May 28, 2021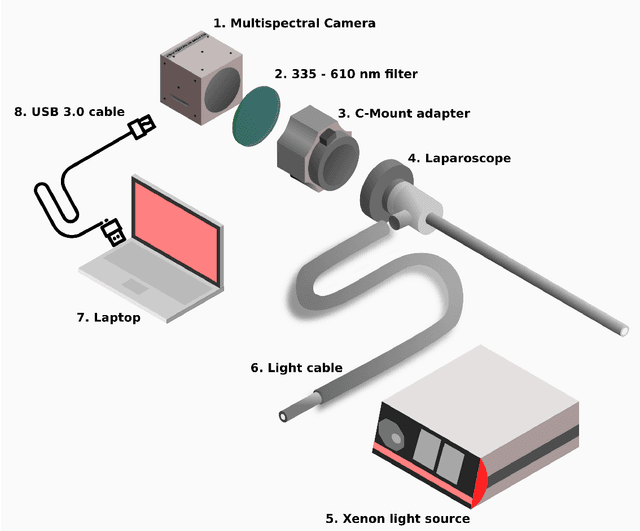
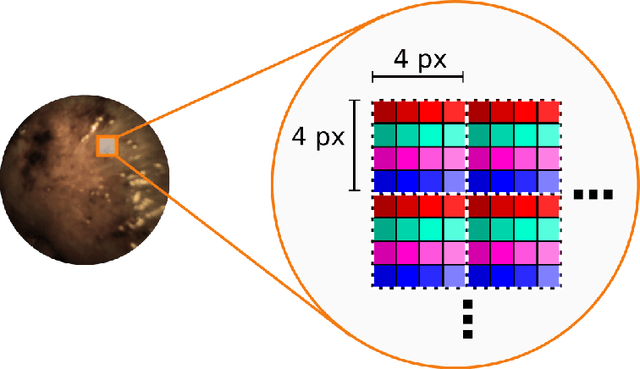
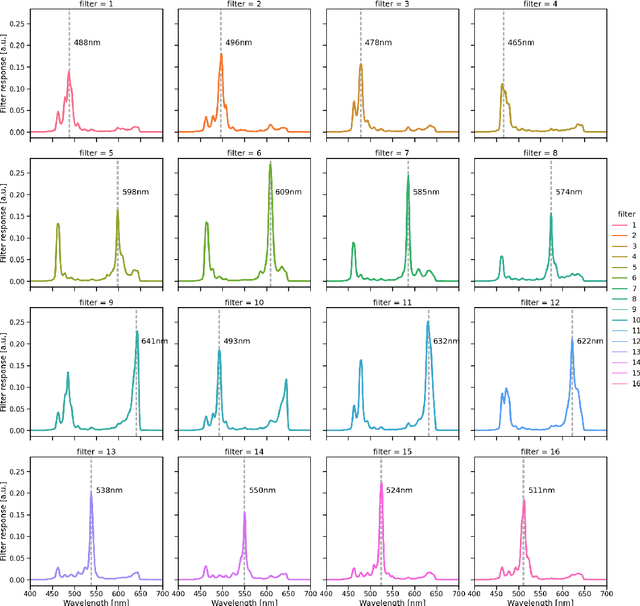
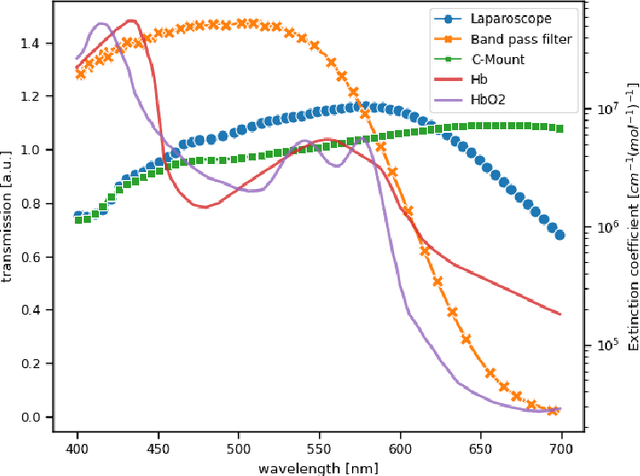
Abstract:Multispectral and hyperspectral imaging (MSI/HSI) can provide clinically relevant information on morphological and functional tissue properties. Application in the operating room (OR), however, has so far been limited by complex hardware setups and slow acquisition times. To overcome these limitations, we propose a novel imaging system for video-rate spectral imaging in the clinical workflow. The system integrates a small snapshot multispectral camera with a standard laparoscope and a clinically commonly used light source, enabling the recording of multispectral images with a spectral dimension of 16 at a frame rate of 25 Hz. An ongoing in patient study shows that multispectral recordings from this system can help detect perfusion changes in partial nephrectomy surgery, thus opening the doors to a wide range of clinical applications.
Out of distribution detection for intra-operative functional imaging
Nov 05, 2019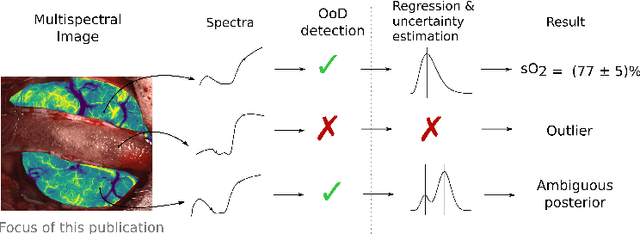
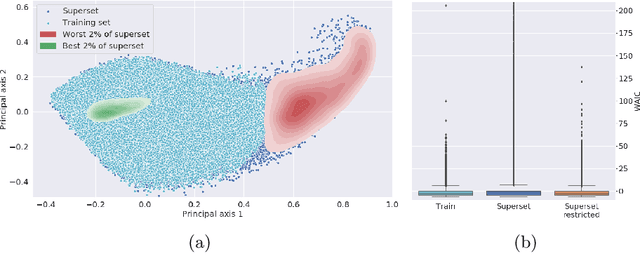
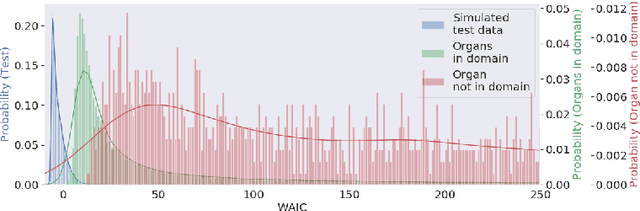
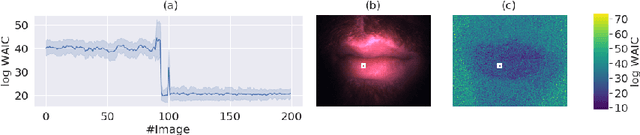
Abstract:Multispectral optical imaging is becoming a key tool in the operating room. Recent research has shown that machine learning algorithms can be used to convert pixel-wise reflectance measurements to tissue parameters, such as oxygenation. However, the accuracy of these algorithms can only be guaranteed if the spectra acquired during surgery match the ones seen during training. It is therefore of great interest to detect so-called out of distribution (OoD) spectra to prevent the algorithm from presenting spurious results. In this paper we present an information theory based approach to OoD detection based on the widely applicable information criterion (WAIC). Our work builds upon recent methodology related to invertible neural networks (INN). Specifically, we make use of an ensemble of INNs as we need their tractable Jacobians in order to compute the WAIC. Comprehensive experiments with in silico, and in vivo multispectral imaging data indicate that our approach is well-suited for OoD detection. Our method could thus be an important step towards reliable functional imaging in the operating room.
* The final authenticated version is available online at https://doi.org/10.1007/978-3-030-32689-0_8
Uncertainty-aware performance assessment of optical imaging modalities with invertible neural networks
Mar 08, 2019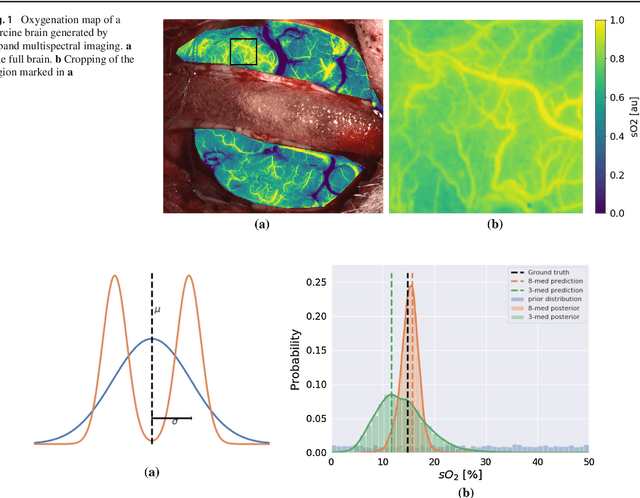
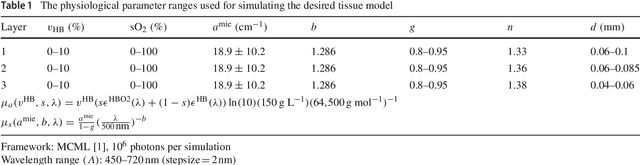

Abstract:Purpose: Optical imaging is evolving as a key technique for advanced sensing in the operating room. Recent research has shown that machine learning algorithms can be used to address the inverse problem of converting pixel-wise multispectral reflectance measurements to underlying tissue parameters, such as oxygenation. Assessment of the specific hardware used in conjunction with such algorithms, however, has not properly addressed the possibility that the problem may be ill-posed. Methods: We present a novel approach to the assessment of optical imaging modalities, which is sensitive to the different types of uncertainties that may occur when inferring tissue parameters. Based on the concept of invertible neural networks, our framework goes beyond point estimates and maps each multispectral measurement to a full posterior probability distribution which is capable of representing ambiguity in the solution via multiple modes. Performance metrics for a hardware setup can then be computed from the characteristics of the posteriors. Results: Application of the assessment framework to the specific use case of camera selection for physiological parameter estimation yields the following insights: (1) Estimation of tissue oxygenation from multispectral images is a well-posed problem, while (2) blood volume fraction may not be recovered without ambiguity. (3) In general, ambiguity may be reduced by increasing the number of spectral bands in the camera. Conclusion: Our method could help to optimize optical camera design in an application-specific manner.
Exploiting the potential of unlabeled endoscopic video data with self-supervised learning
Jan 31, 2018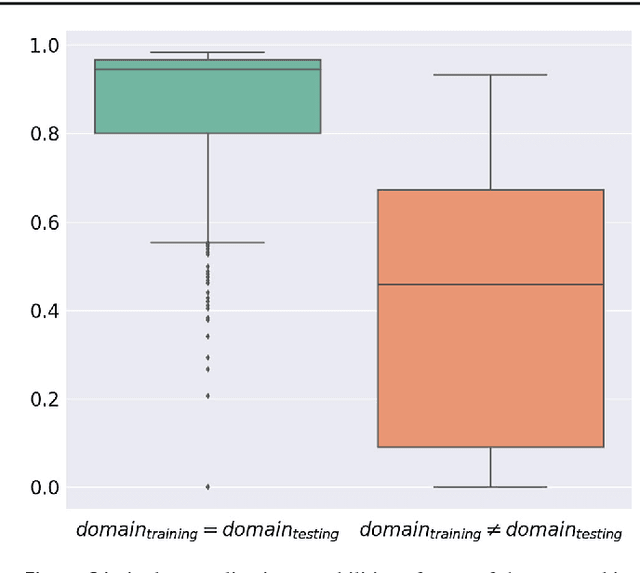
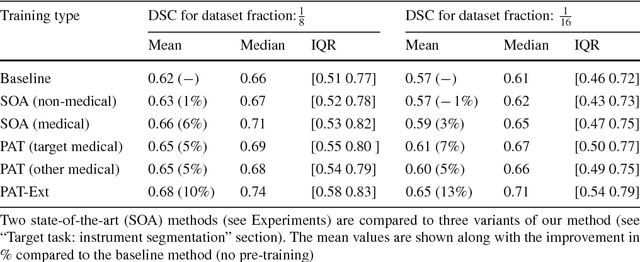
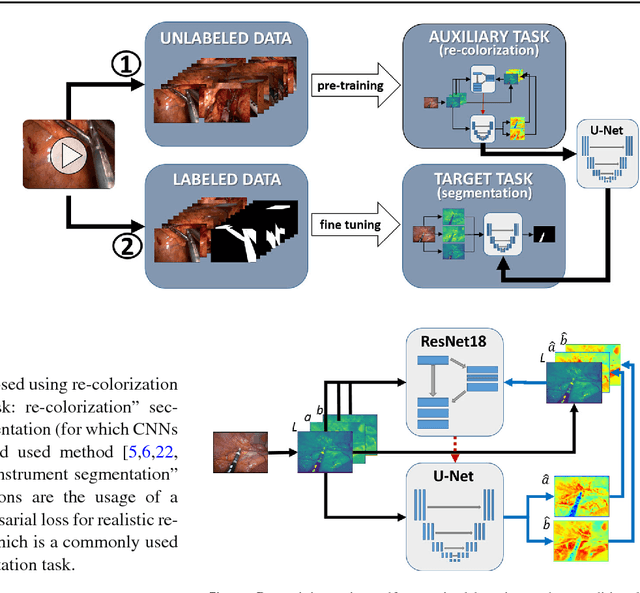
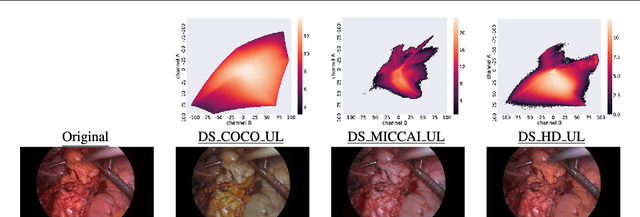
Abstract:Surgical data science is a new research field that aims to observe all aspects of the patient treatment process in order to provide the right assistance at the right time. Due to the breakthrough successes of deep learning-based solutions for automatic image annotation, the availability of reference annotations for algorithm training is becoming a major bottleneck in the field. The purpose of this paper was to investigate the concept of self-supervised learning to address this issue. Our approach is guided by the hypothesis that unlabeled video data can be used to learn a representation of the target domain that boosts the performance of state-of-the-art machine learning algorithms when used for pre-training. Core of the method is an auxiliary task based on raw endoscopic video data of the target domain that is used to initialize the convolutional neural network (CNN) for the target task. In this paper, we propose the re-colorization of medical images with a generative adversarial network (GAN)-based architecture as auxiliary task. A variant of the method involves a second pre-training step based on labeled data for the target task from a related domain. We validate both variants using medical instrument segmentation as target task. The proposed approach can be used to radically reduce the manual annotation effort involved in training CNNs. Compared to the baseline approach of generating annotated data from scratch, our method decreases exploratively the number of labeled images by up to 75% without sacrificing performance. Our method also outperforms alternative methods for CNN pre-training, such as pre-training on publicly available non-medical or medical data using the target task (in this instance: segmentation). As it makes efficient use of available (non-)public and (un-)labeled data, the approach has the potential to become a valuable tool for CNN (pre-)training.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge