Zekun Guo
Beyond Rule-Based Workflows: An Information-Flow-Orchestrated Multi-Agents Paradigm via Agent-to-Agent Communication from CORAL
Jan 14, 2026Abstract:Most existing Large Language Model (LLM)-based Multi-Agent Systems (MAS) rely on predefined workflows, where human engineers enumerate task states in advance and specify routing rules and contextual injections accordingly. Such workflow-driven designs are essentially rule-based decision trees, which suffer from two fundamental limitations: they require substantial manual effort to anticipate and encode possible task states, and they cannot exhaustively cover the state space of complex real-world tasks. To address these issues, we propose an Information-Flow-Orchestrated Multi-Agent Paradigm via Agent-to-Agent (A2A) Communication from CORAL, in which a dedicated information flow orchestrator continuously monitors task progress and dynamically coordinates other agents through the A2A toolkit using natural language, without relying on predefined workflows. We evaluate our approach on the general-purpose benchmark GAIA, using the representative workflow-based MAS OWL as the baseline while controlling for agent roles and underlying models. Under the pass@1 setting, our method achieves 63.64% accuracy, outperforming OWL's 55.15% by 8.49 percentage points with comparable token consumption. Further case-level analysis shows that our paradigm enables more flexible task monitoring and more robust handling of edge cases. Our implementation is publicly available at: https://github.com/Coral-Protocol/Beyond-Rule-Based-Workflows
MolChord: Structure-Sequence Alignment for Protein-Guided Drug Design
Oct 31, 2025Abstract:Structure-based drug design (SBDD), which maps target proteins to candidate molecular ligands, is a fundamental task in drug discovery. Effectively aligning protein structural representations with molecular representations, and ensuring alignment between generated drugs and their pharmacological properties, remains a critical challenge. To address these challenges, we propose MolChord, which integrates two key techniques: (1) to align protein and molecule structures with their textual descriptions and sequential representations (e.g., FASTA for proteins and SMILES for molecules), we leverage NatureLM, an autoregressive model unifying text, small molecules, and proteins, as the molecule generator, alongside a diffusion-based structure encoder; and (2) to guide molecules toward desired properties, we curate a property-aware dataset by integrating preference data and refine the alignment process using Direct Preference Optimization (DPO). Experimental results on CrossDocked2020 demonstrate that our approach achieves state-of-the-art performance on key evaluation metrics, highlighting its potential as a practical tool for SBDD.
Anemoi: A Semi-Centralized Multi-agent Systems Based on Agent-to-Agent Communication MCP server from Coral Protocol
Aug 23, 2025Abstract:Recent advances in generalist multi-agent systems (MAS) have largely followed a context-engineering plus centralized paradigm, where a planner agent coordinates multiple worker agents through unidirectional prompt passing. While effective under strong planner models, this design suffers from two critical limitations: (1) strong dependency on the planner's capability, which leads to degraded performance when a smaller LLM powers the planner; and (2) limited inter-agent communication, where collaboration relies on costly prompt concatenation and context injection, introducing redundancy and information loss. To address these challenges, we propose Anemoi, a semi-centralized MAS built on the Agent-to-Agent (A2A) communication MCP server from Coral Protocol. Unlike traditional designs, Anemoi enables structured and direct inter-agent collaboration, allowing all agents to monitor progress, assess results, identify bottlenecks, and propose refinements in real time. This paradigm reduces reliance on a single planner, supports adaptive plan updates, and minimizes redundant context passing, resulting in more scalable and cost-efficient execution. Evaluated on the GAIA benchmark, Anemoi achieved 52.73\% accuracy with a small LLM (GPT-4.1-mini) as the planner, surpassing the strongest open-source baseline OWL (43.63\%) by +9.09\% under identical LLM settings. Our implementation is publicly available at https://github.com/Coral-Protocol/Anemoi.
NatureLM: Deciphering the Language of Nature for Scientific Discovery
Feb 11, 2025



Abstract:Foundation models have revolutionized natural language processing and artificial intelligence, significantly enhancing how machines comprehend and generate human languages. Inspired by the success of these foundation models, researchers have developed foundation models for individual scientific domains, including small molecules, materials, proteins, DNA, and RNA. However, these models are typically trained in isolation, lacking the ability to integrate across different scientific domains. Recognizing that entities within these domains can all be represented as sequences, which together form the "language of nature", we introduce Nature Language Model (briefly, NatureLM), a sequence-based science foundation model designed for scientific discovery. Pre-trained with data from multiple scientific domains, NatureLM offers a unified, versatile model that enables various applications including: (i) generating and optimizing small molecules, proteins, RNA, and materials using text instructions; (ii) cross-domain generation/design, such as protein-to-molecule and protein-to-RNA generation; and (iii) achieving state-of-the-art performance in tasks like SMILES-to-IUPAC translation and retrosynthesis on USPTO-50k. NatureLM offers a promising generalist approach for various scientific tasks, including drug discovery (hit generation/optimization, ADMET optimization, synthesis), novel material design, and the development of therapeutic proteins or nucleotides. We have developed NatureLM models in different sizes (1 billion, 8 billion, and 46.7 billion parameters) and observed a clear improvement in performance as the model size increases.
Regulator-Manufacturer AI Agents Modeling: Mathematical Feedback-Driven Multi-Agent LLM Framework
Nov 22, 2024



Abstract:The increasing complexity of regulatory updates from global authorities presents significant challenges for medical device manufacturers, necessitating agile strategies to sustain compliance and maintain market access. Concurrently, regulatory bodies must effectively monitor manufacturers' responses and develop strategic surveillance plans. This study employs a multi-agent modeling approach, enhanced with Large Language Models (LLMs), to simulate regulatory dynamics and examine the adaptive behaviors of key actors, including regulatory bodies, manufacturers, and competitors. These agents operate within a simulated environment governed by regulatory flow theory, capturing the impacts of regulatory changes on compliance decisions, market adaptation, and innovation strategies. Our findings illuminate the influence of regulatory shifts on industry behaviour and identify strategic opportunities for improving regulatory practices, optimizing compliance, and fostering innovation. By leveraging the integration of multi-agent systems and LLMs, this research provides a novel perspective and offers actionable insights for stakeholders navigating the evolving regulatory landscape of the medical device industry.
NTIRE 2023 Quality Assessment of Video Enhancement Challenge
Jul 19, 2023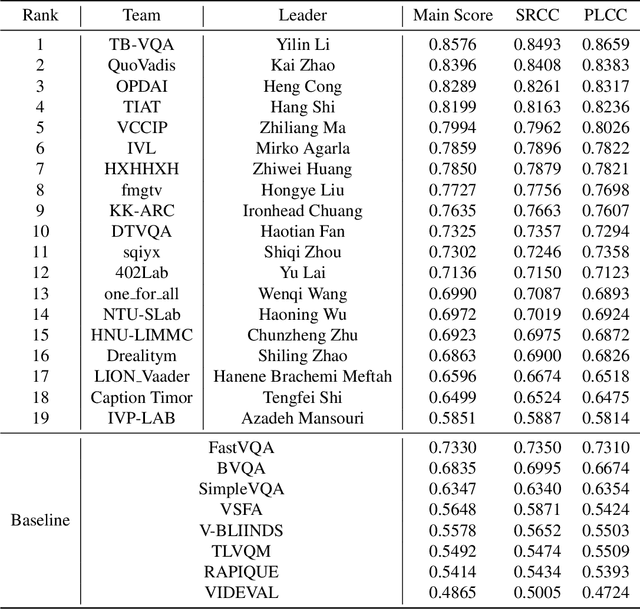
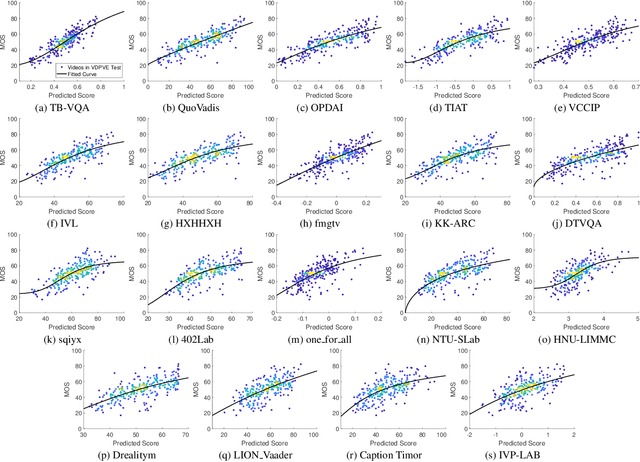

Abstract:This paper reports on the NTIRE 2023 Quality Assessment of Video Enhancement Challenge, which will be held in conjunction with the New Trends in Image Restoration and Enhancement Workshop (NTIRE) at CVPR 2023. This challenge is to address a major challenge in the field of video processing, namely, video quality assessment (VQA) for enhanced videos. The challenge uses the VQA Dataset for Perceptual Video Enhancement (VDPVE), which has a total of 1211 enhanced videos, including 600 videos with color, brightness, and contrast enhancements, 310 videos with deblurring, and 301 deshaked videos. The challenge has a total of 167 registered participants. 61 participating teams submitted their prediction results during the development phase, with a total of 3168 submissions. A total of 176 submissions were submitted by 37 participating teams during the final testing phase. Finally, 19 participating teams submitted their models and fact sheets, and detailed the methods they used. Some methods have achieved better results than baseline methods, and the winning methods have demonstrated superior prediction performance.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge