Xuemei Wang
Towards Globally Predictable k-Space Interpolation: A White-box Transformer Approach
Aug 06, 2025Abstract:Interpolating missing data in k-space is essential for accelerating imaging. However, existing methods, including convolutional neural network-based deep learning, primarily exploit local predictability while overlooking the inherent global dependencies in k-space. Recently, Transformers have demonstrated remarkable success in natural language processing and image analysis due to their ability to capture long-range dependencies. This inspires the use of Transformers for k-space interpolation to better exploit its global structure. However, their lack of interpretability raises concerns regarding the reliability of interpolated data. To address this limitation, we propose GPI-WT, a white-box Transformer framework based on Globally Predictable Interpolation (GPI) for k-space. Specifically, we formulate GPI from the perspective of annihilation as a novel k-space structured low-rank (SLR) model. The global annihilation filters in the SLR model are treated as learnable parameters, and the subgradients of the SLR model naturally induce a learnable attention mechanism. By unfolding the subgradient-based optimization algorithm of SLR into a cascaded network, we construct the first white-box Transformer specifically designed for accelerated MRI. Experimental results demonstrate that the proposed method significantly outperforms state-of-the-art approaches in k-space interpolation accuracy while providing superior interpretability.
st-DTPM: Spatial-Temporal Guided Diffusion Transformer Probabilistic Model for Delayed Scan PET Image Prediction
Oct 30, 2024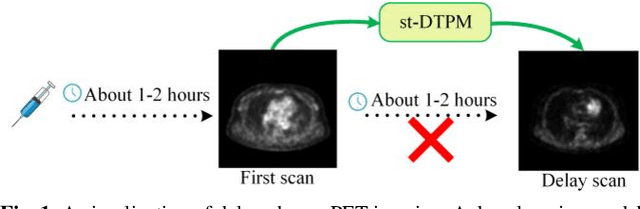
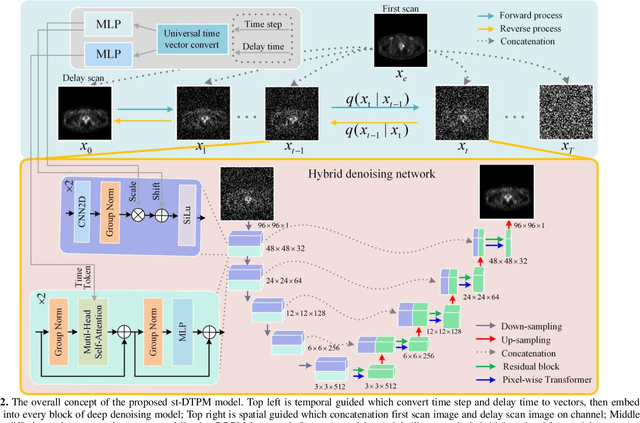
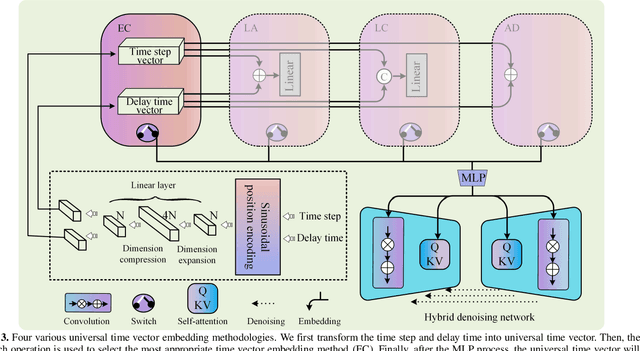

Abstract:PET imaging is widely employed for observing biological metabolic activities within the human body. However, numerous benign conditions can cause increased uptake of radiopharmaceuticals, confounding differentiation from malignant tumors. Several studies have indicated that dual-time PET imaging holds promise in distinguishing between malignant and benign tumor processes. Nevertheless, the hour-long distribution period of radiopharmaceuticals post-injection complicates the determination of optimal timing for the second scan, presenting challenges in both practical applications and research. Notably, we have identified that delay time PET imaging can be framed as an image-to-image conversion problem. Motivated by this insight, we propose a novel spatial-temporal guided diffusion transformer probabilistic model (st-DTPM) to solve dual-time PET imaging prediction problem. Specifically, this architecture leverages the U-net framework that integrates patch-wise features of CNN and pixel-wise relevance of Transformer to obtain local and global information. And then employs a conditional DDPM model for image synthesis. Furthermore, on spatial condition, we concatenate early scan PET images and noisy PET images on every denoising step to guide the spatial distribution of denoising sampling. On temporal condition, we convert diffusion time steps and delay time to a universal time vector, then embed it to each layer of model architecture to further improve the accuracy of predictions. Experimental results demonstrated the superiority of our method over alternative approaches in preserving image quality and structural information, thereby affirming its efficacy in predictive task.
Joint PET-MRI Reconstruction with Diffusion Stochastic Differential Model
Aug 07, 2024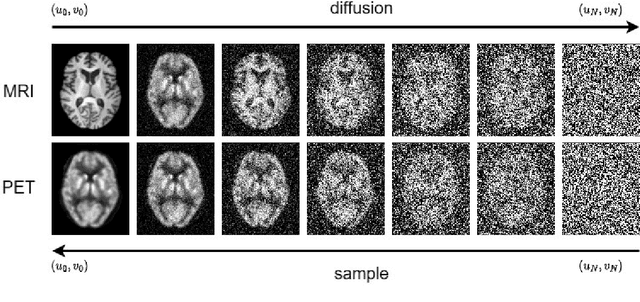
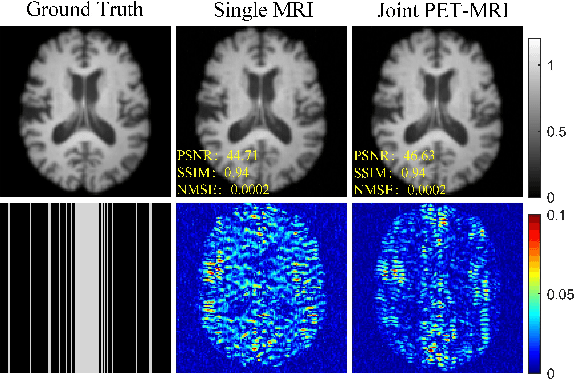
Abstract:PET suffers from a low signal-to-noise ratio. Meanwhile, the k-space data acquisition process in MRI is time-consuming by PET-MRI systems. We aim to accelerate MRI and improve PET image quality. This paper proposed a novel joint reconstruction model by diffusion stochastic differential equations based on learning the joint probability distribution of PET and MRI. Compare the results underscore the qualitative and quantitative improvements our model brings to PET and MRI reconstruction, surpassing the current state-of-the-art methodologies. Joint PET-MRI reconstruction is a challenge in the PET-MRI system. This studies focused on the relationship extends beyond edges. In this study, PET is generated from MRI by learning joint probability distribution as the relationship.
* Accepted as ISMRM 2024 Digital poster 6575. 04-09 May 2024 Singapore
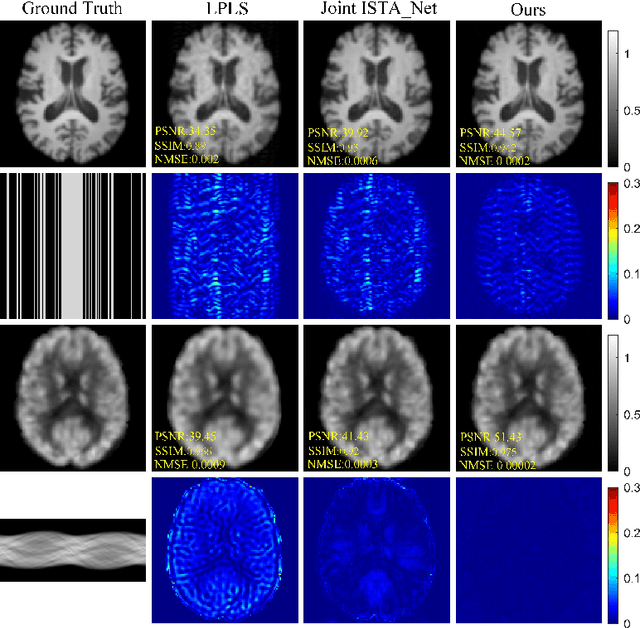
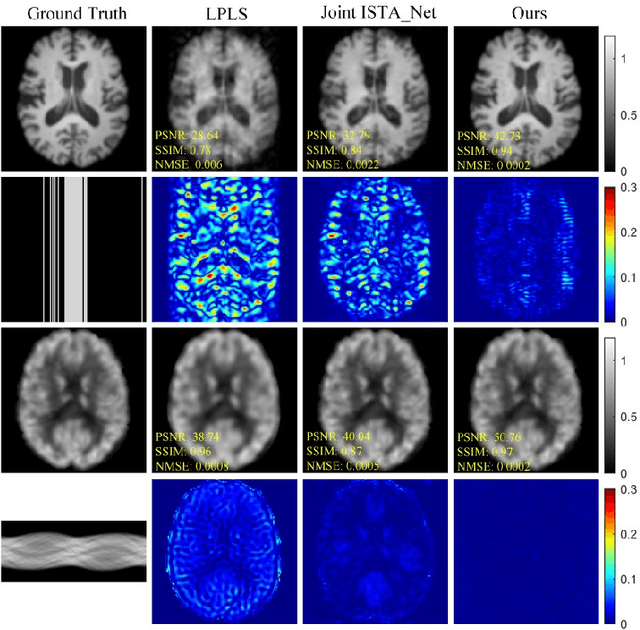
Joint Diffusion: Mutual Consistency-Driven Diffusion Model for PET-MRI Co-Reconstruction
Nov 24, 2023Abstract:Positron Emission Tomography and Magnetic Resonance Imaging (PET-MRI) systems can obtain functional and anatomical scans. PET suffers from a low signal-to-noise ratio. Meanwhile, the k-space data acquisition process in MRI is time-consuming. The study aims to accelerate MRI and enhance PET image quality. Conventional approaches involve the separate reconstruction of each modality within PET-MRI systems. However, there exists complementary information among multi-modal images. The complementary information can contribute to image reconstruction. In this study, we propose a novel PET-MRI joint reconstruction model employing a mutual consistency-driven diffusion mode, namely MC-Diffusion. MC-Diffusion learns the joint probability distribution of PET and MRI for utilizing complementary information. We conducted a series of contrast experiments about LPLS, Joint ISAT-net and MC-Diffusion by the ADNI dataset. The results underscore the qualitative and quantitative improvements achieved by MC-Diffusion, surpassing the state-of-the-art method.
Deep learning radiomics for assessment of gastroesophageal varices in people with compensated advanced chronic liver disease
Jun 13, 2023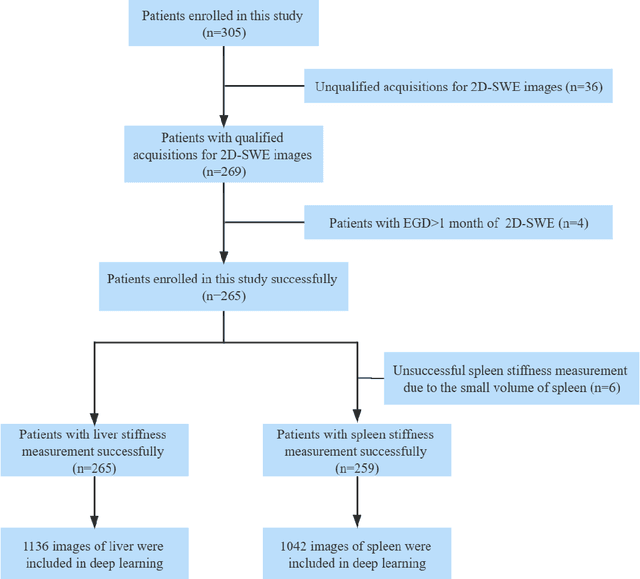
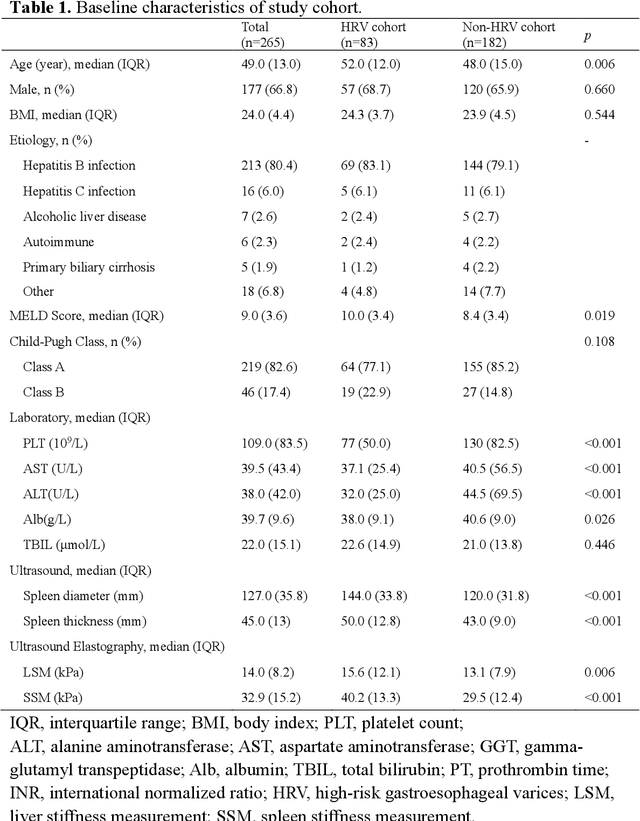
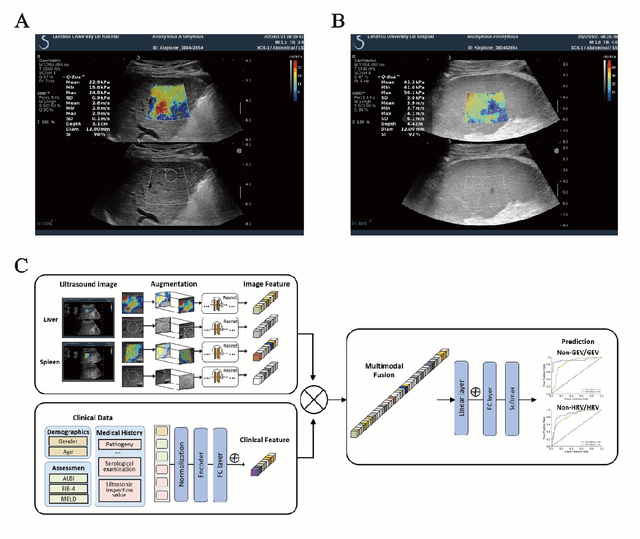
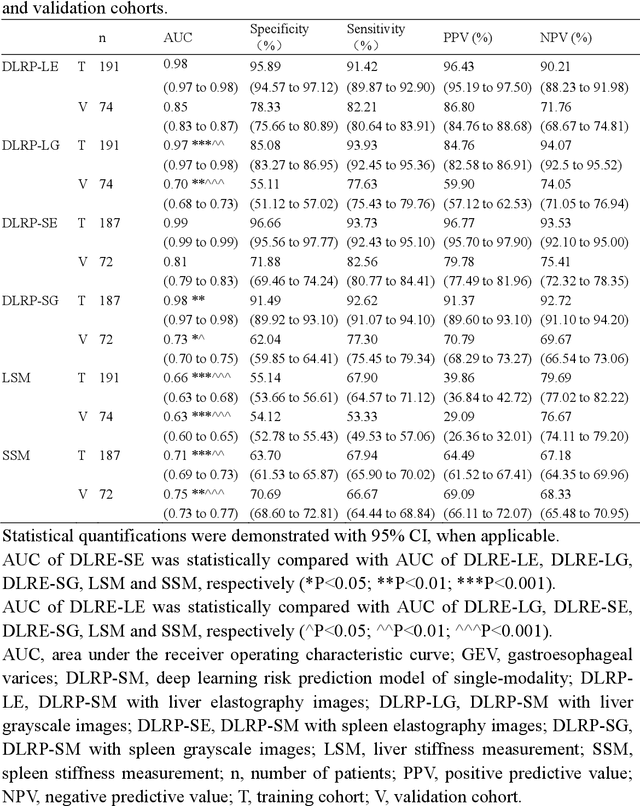
Abstract:Objective: Bleeding from gastroesophageal varices (GEV) is a medical emergency associated with high mortality. We aim to construct an artificial intelligence-based model of two-dimensional shear wave elastography (2D-SWE) of the liver and spleen to precisely assess the risk of GEV and high-risk gastroesophageal varices (HRV). Design: A prospective multicenter study was conducted in patients with compensated advanced chronic liver disease. 305 patients were enrolled from 12 hospitals, and finally 265 patients were included, with 1136 liver stiffness measurement (LSM) images and 1042 spleen stiffness measurement (SSM) images generated by 2D-SWE. We leveraged deep learning methods to uncover associations between image features and patient risk, and thus conducted models to predict GEV and HRV. Results: A multi-modality Deep Learning Risk Prediction model (DLRP) was constructed to assess GEV and HRV, based on LSM and SSM images, and clinical information. Validation analysis revealed that the AUCs of DLRP were 0.91 for GEV (95% CI 0.90 to 0.93, p < 0.05) and 0.88 for HRV (95% CI 0.86 to 0.89, p < 0.01), which were significantly and robustly better than canonical risk indicators, including the value of LSM and SSM. Moreover, DLPR was better than the model using individual parameters, including LSM and SSM images. In HRV prediction, the 2D-SWE images of SSM outperform LSM (p < 0.01). Conclusion: DLRP shows excellent performance in predicting GEV and HRV over canonical risk indicators LSM and SSM. Additionally, the 2D-SWE images of SSM provided more information for better accuracy in predicting HRV than the LSM.
Synthesizing PET images from High-field and Ultra-high-field MR images Using Joint Diffusion Attention Model
May 06, 2023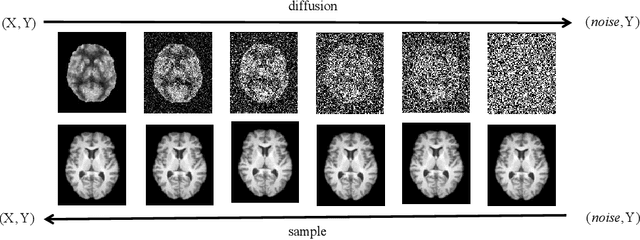
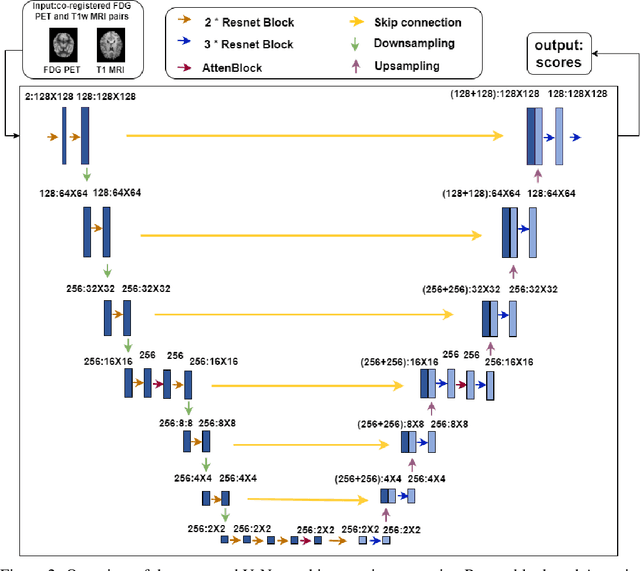
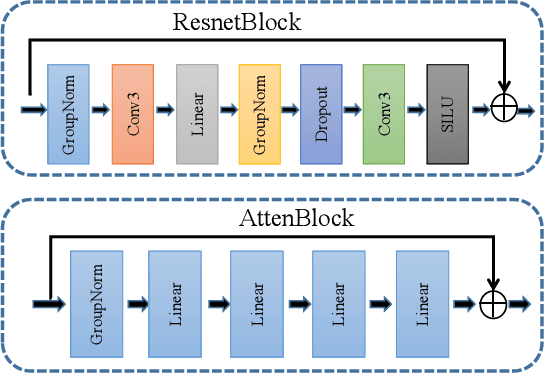
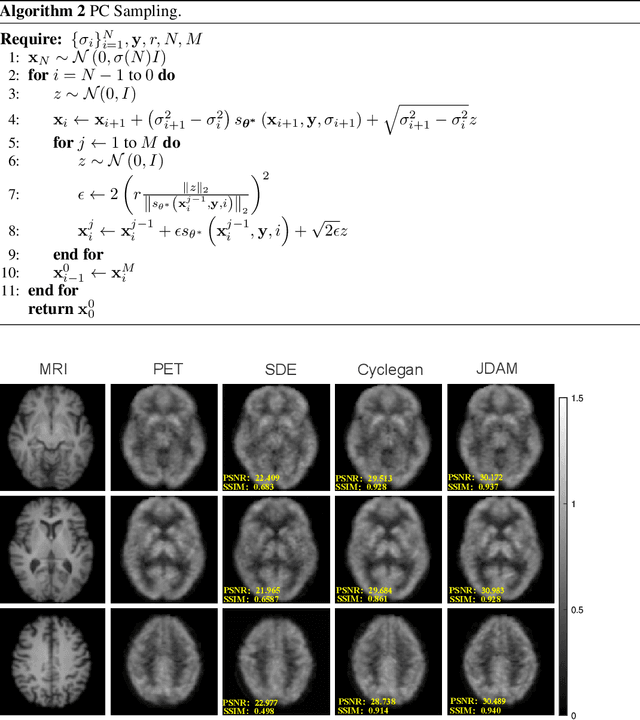
Abstract:MRI and PET are crucial diagnostic tools for brain diseases, as they provide complementary information on brain structure and function. However, PET scanning is costly and involves radioactive exposure, resulting in a lack of PET. Moreover, simultaneous PET and MRI at ultra-high-field are currently hardly infeasible. Ultra-high-field imaging has unquestionably proven valuable in both clinical and academic settings, especially in the field of cognitive neuroimaging. These motivate us to propose a method for synthetic PET from high-filed MRI and ultra-high-field MRI. From a statistical perspective, the joint probability distribution (JPD) is the most direct and fundamental means of portraying the correlation between PET and MRI. This paper proposes a novel joint diffusion attention model which has the joint probability distribution and attention strategy, named JDAM. JDAM has a diffusion process and a sampling process. The diffusion process involves the gradual diffusion of PET to Gaussian noise by adding Gaussian noise, while MRI remains fixed. JPD of MRI and noise-added PET was learned in the diffusion process. The sampling process is a predictor-corrector. PET images were generated from MRI by JPD of MRI and noise-added PET. The predictor is a reverse diffusion process and the corrector is Langevin dynamics. Experimental results on the public Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset demonstrate that the proposed method outperforms state-of-the-art CycleGAN for high-field MRI (3T MRI). Finally, synthetic PET images from the ultra-high-field (5T MRI and 7T MRI) be attempted, providing a possibility for ultra-high-field PET-MRI imaging.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge