Wei-Chieh Huang
From Web Search towards Agentic Deep Research: Incentivizing Search with Reasoning Agents
Jun 23, 2025Abstract:Information retrieval is a cornerstone of modern knowledge acquisition, enabling billions of queries each day across diverse domains. However, traditional keyword-based search engines are increasingly inadequate for handling complex, multi-step information needs. Our position is that Large Language Models (LLMs), endowed with reasoning and agentic capabilities, are ushering in a new paradigm termed Agentic Deep Research. These systems transcend conventional information search techniques by tightly integrating autonomous reasoning, iterative retrieval, and information synthesis into a dynamic feedback loop. We trace the evolution from static web search to interactive, agent-based systems that plan, explore, and learn. We also introduce a test-time scaling law to formalize the impact of computational depth on reasoning and search. Supported by benchmark results and the rise of open-source implementations, we demonstrate that Agentic Deep Research not only significantly outperforms existing approaches, but is also poised to become the dominant paradigm for future information seeking. All the related resources, including industry products, research papers, benchmark datasets, and open-source implementations, are collected for the community in https://github.com/DavidZWZ/Awesome-Deep-Research.
A Call for Collaborative Intelligence: Why Human-Agent Systems Should Precede AI Autonomy
Jun 11, 2025Abstract:Recent improvements in large language models (LLMs) have led many researchers to focus on building fully autonomous AI agents. This position paper questions whether this approach is the right path forward, as these autonomous systems still have problems with reliability, transparency, and understanding the actual requirements of human. We suggest a different approach: LLM-based Human-Agent Systems (LLM-HAS), where AI works with humans rather than replacing them. By keeping human involved to provide guidance, answer questions, and maintain control, these systems can be more trustworthy and adaptable. Looking at examples from healthcare, finance, and software development, we show how human-AI teamwork can handle complex tasks better than AI working alone. We also discuss the challenges of building these collaborative systems and offer practical solutions. This paper argues that progress in AI should not be measured by how independent systems become, but by how well they can work with humans. The most promising future for AI is not in systems that take over human roles, but in those that enhance human capabilities through meaningful partnership.
A Survey on Large Language Model based Human-Agent Systems
May 01, 2025Abstract:Recent advances in large language models (LLMs) have sparked growing interest in building fully autonomous agents. However, fully autonomous LLM-based agents still face significant challenges, including limited reliability due to hallucinations, difficulty in handling complex tasks, and substantial safety and ethical risks, all of which limit their feasibility and trustworthiness in real-world applications. To overcome these limitations, LLM-based human-agent systems (LLM-HAS) incorporate human-provided information, feedback, or control into the agent system to enhance system performance, reliability and safety. This paper provides the first comprehensive and structured survey of LLM-HAS. It clarifies fundamental concepts, systematically presents core components shaping these systems, including environment & profiling, human feedback, interaction types, orchestration and communication, explores emerging applications, and discusses unique challenges and opportunities. By consolidating current knowledge and offering a structured overview, we aim to foster further research and innovation in this rapidly evolving interdisciplinary field. Paper lists and resources are available at https://github.com/HenryPengZou/Awesome-LLM-Based-Human-Agent-System-Papers.
Federated Learning for Coronary Artery Plaque Detection in Atherosclerosis Using IVUS Imaging: A Multi-Hospital Collaboration
Dec 19, 2024Abstract:The traditional interpretation of Intravascular Ultrasound (IVUS) images during Percutaneous Coronary Intervention (PCI) is time-intensive and inconsistent, relying heavily on physician expertise. Regulatory restrictions and privacy concerns further hinder data integration across hospital systems, complicating collaborative analysis. To address these challenges, a parallel 2D U-Net model with a multi-stage segmentation architecture has been developed, utilizing federated learning to enable secure data analysis across institutions while preserving privacy. The model segments plaques by identifying and subtracting the External Elastic Membrane (EEM) and lumen areas, with preprocessing converting Cartesian to polar coordinates for improved computational efficiency. Achieving a Dice Similarity Coefficient (DSC) of 0.706, the model effectively identifies plaques and detects circular boundaries in real-time. Collaborative efforts with domain experts enhance plaque burden interpretation through precise quantitative measurements. Future advancements may involve integrating advanced federated learning techniques and expanding datasets to further improve performance and applicability. This adaptable technology holds promise for environments handling sensitive, distributed data, offering potential to optimize outcomes in medical imaging and intervention.
Self-supervised based general laboratory progress pretrained model for cardiovascular event detection
Mar 15, 2023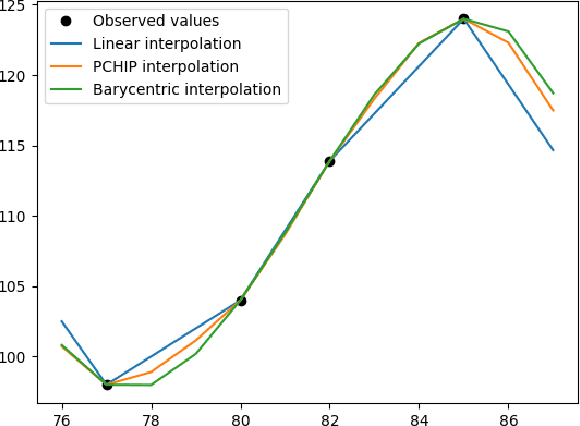
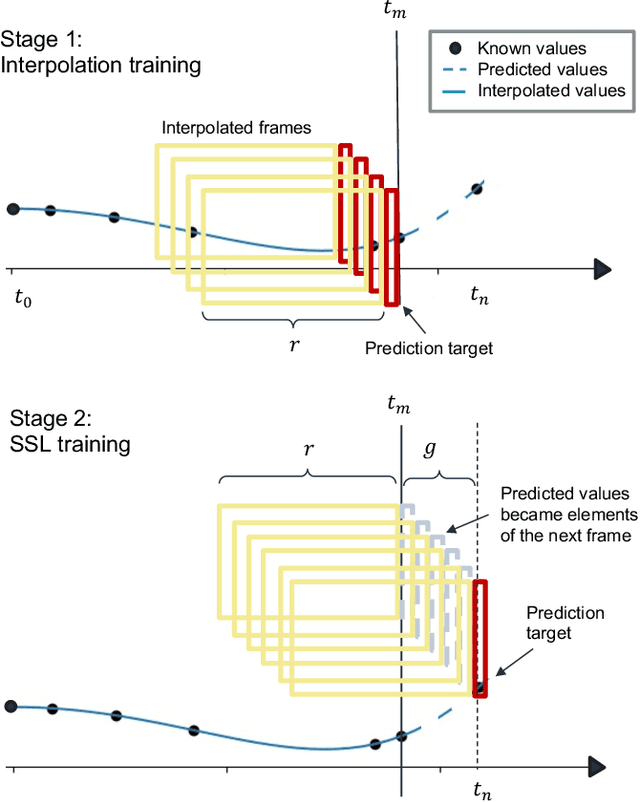
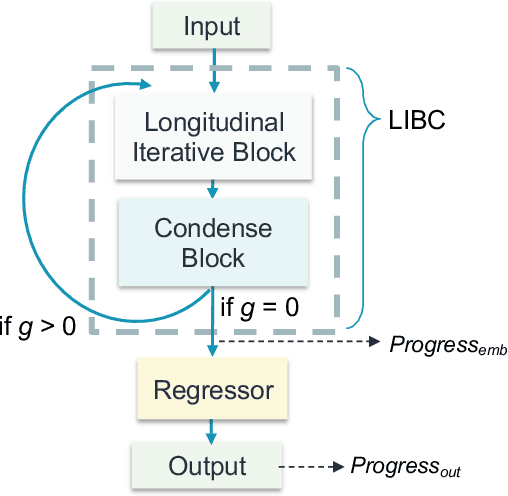
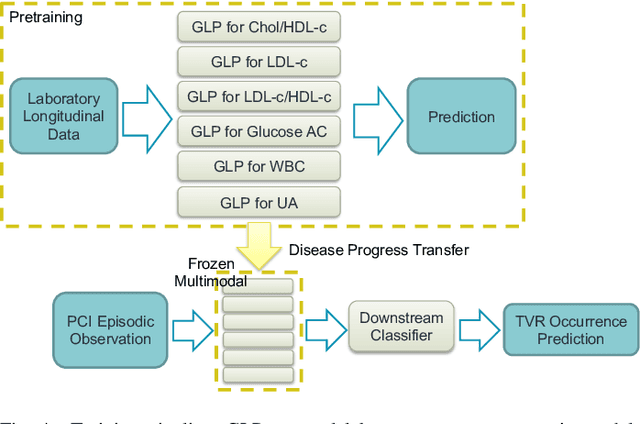
Abstract:Regular surveillance is an indispensable aspect of managing cardiovascular disorders. Patient recruitment for rare or specific diseases is often limited due to their small patient size and episodic observations, whereas prevalent cases accumulate longitudinal data easily due to regular follow-ups. These data, however, are notorious for their irregularity, temporality, absenteeism, and sparsity. In this study, we leveraged self-supervised learning (SSL) and transfer learning to overcome the above-mentioned barriers, transferring patient progress trends in cardiovascular laboratory parameters from prevalent cases to rare or specific cardiovascular events detection. We pretrained a general laboratory progress (GLP) pretrain model using hypertension patients (who were yet to be diabetic), and transferred their laboratory progress trend to assist in detecting target vessel revascularization (TVR) in percutaneous coronary intervention patients. GLP adopted a two-stage training process that utilized interpolated data, enhancing the performance of SSL. After pretraining GLP, we fine-tuned it for TVR prediction. The proposed two-stage training process outperformed SSL. Upon processing by GLP, the classification demonstrated a marked improvement, increasing from 0.63 to 0.90 in averaged accuracy. All metrics were significantly superior (p < 0.01) to the performance of prior GLP processing. The representation displayed distinct separability independent of algorithmic mechanisms, and diverse data distribution trend. Our approach effectively transferred the progression trends of cardiovascular laboratory parameters from prevalent cases to small-numbered cases, thereby demonstrating its efficacy in aiding the risk assessment of cardiovascular events without limiting to episodic observation. The potential for extending this approach to other laboratory tests and diseases is promising.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge