Shamim Nemati
Improving Prediction of Need for Mechanical Ventilation using Cross-Attention
Jul 21, 2024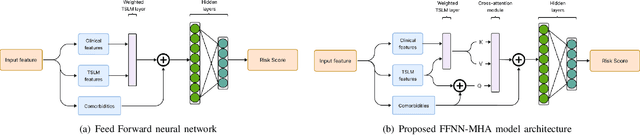
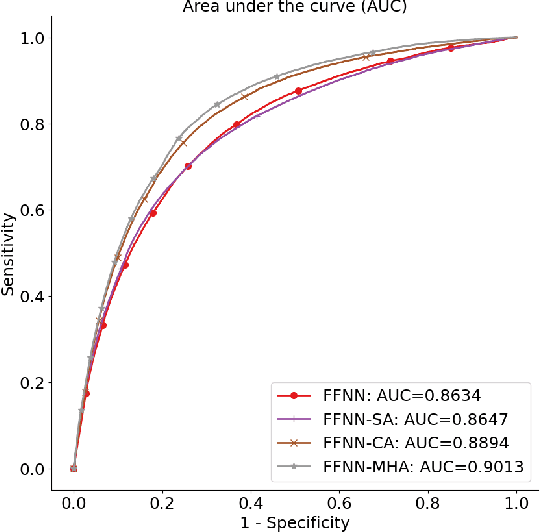
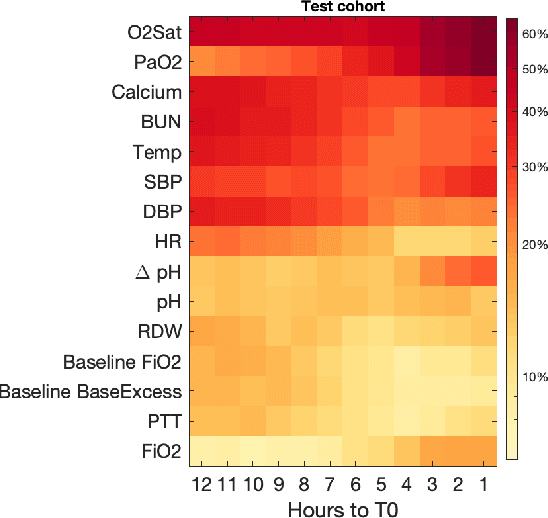
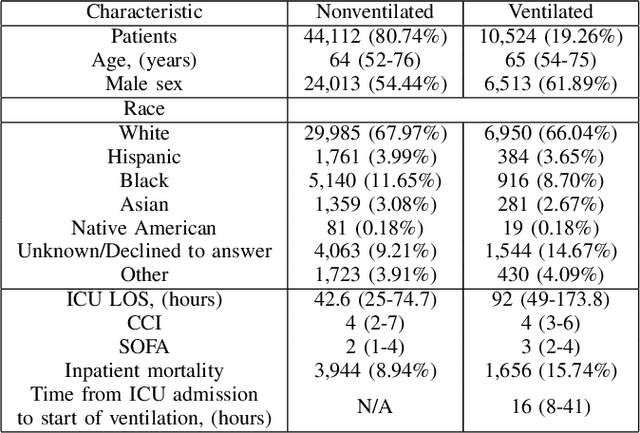
Abstract:In the intensive care unit, the capability to predict the need for mechanical ventilation (MV) facilitates more timely interventions to improve patient outcomes. Recent works have demonstrated good performance in this task utilizing machine learning models. This paper explores the novel application of a deep learning model with multi-head attention (FFNN-MHA) to make more accurate MV predictions and reduce false positives by learning personalized contextual information of individual patients. Utilizing the publicly available MIMIC-IV dataset, FFNN-MHA demonstrates an improvement of 0.0379 in AUC and a 17.8\% decrease in false positives compared to baseline models such as feed-forward neural networks. Our results highlight the potential of the FFNN-MHA model as an effective tool for accurate prediction of the need for mechanical ventilation in critical care settings.
Benchmarking changepoint detection algorithms on cardiac time series
Apr 16, 2024
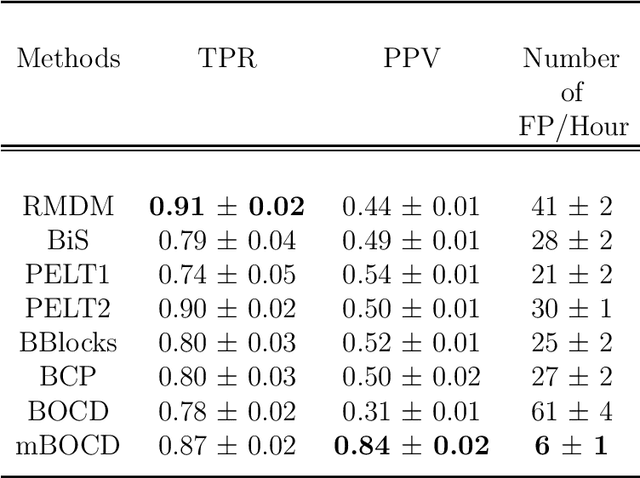

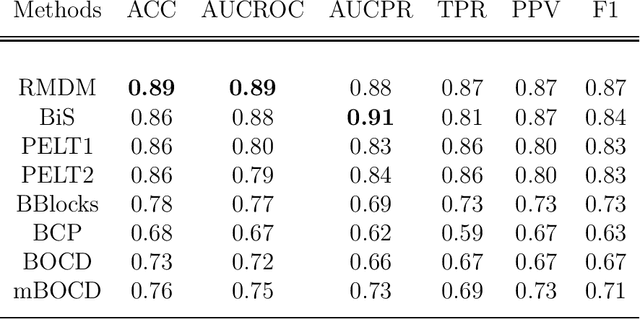
Abstract:The pattern of state changes in a biomedical time series can be related to health or disease. This work presents a principled approach for selecting a changepoint detection algorithm for a specific task, such as disease classification. Eight key algorithms were compared, and the performance of each algorithm was evaluated as a function of temporal tolerance, noise, and abnormal conduction (ectopy) on realistic artificial cardiovascular time series data. All algorithms were applied to real data (cardiac time series of 22 patients with REM-behavior disorder (RBD) and 15 healthy controls) using the parameters selected on artificial data. Finally, features were derived from the detected changepoints to classify RBD patients from healthy controls using a K-Nearest Neighbors approach. On artificial data, Modified Bayesian Changepoint Detection algorithm provided superior positive predictive value for state change identification while Recursive Mean Difference Maximization (RMDM) achieved the highest true positive rate. For the classification task, features derived from the RMDM algorithm provided the highest leave one out cross validated accuracy of 0.89 and true positive rate of 0.87. Automatically detected changepoints provide useful information about subject's physiological state which cannot be directly observed. However, the choice of change point detection algorithm depends on the nature of the underlying data and the downstream application, such as a classification task. This work represents the first time change point detection algorithms have been compared in a meaningful way and utilized in a classification task, which demonstrates the effect of changepoint algorithm choice on application performance.
An Analysis Of Protected Health Information Leakage In Deep-Learning Based De-Identification Algorithms
Jan 28, 2021
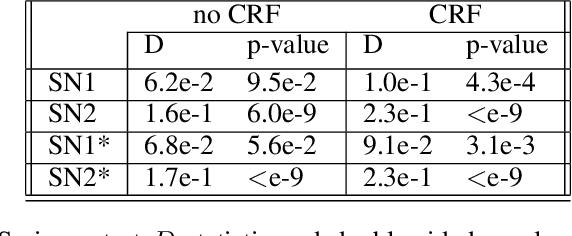
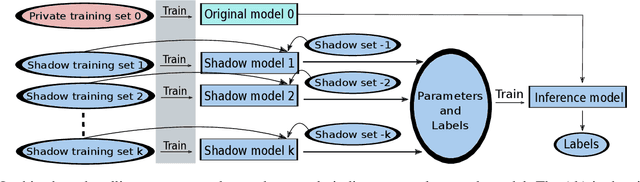
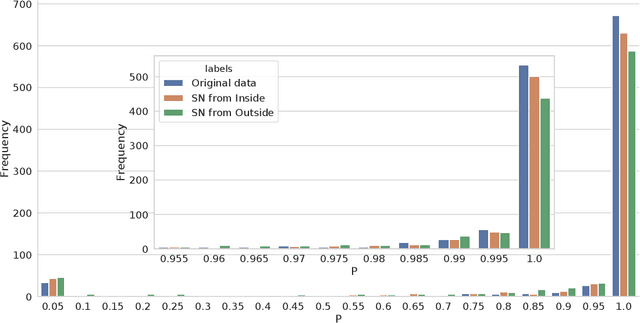
Abstract:The increasing complexity of algorithms for analyzing medical data, including de-identification tasks, raises the possibility that complex algorithms are learning not just the general representation of the problem, but specifics of given individuals within the data. Modern legal frameworks specifically prohibit the intentional or accidental distribution of patient data, but have not addressed this potential avenue for leakage of such protected health information. Modern deep learning algorithms have the highest potential of such leakage due to complexity of the models. Recent research in the field has highlighted such issues in non-medical data, but all analysis is likely to be data and algorithm specific. We, therefore, chose to analyze a state-of-the-art free-text de-identification algorithm based on LSTM (Long Short-Term Memory) and its potential in encoding any individual in the training set. Using the i2b2 Challenge Data, we trained, then analyzed the model to assess whether the output of the LSTM, before the compression layer of the classifier, could be used to estimate the membership of the training data. Furthermore, we used different attacks including membership inference attack method to attack the model. Results indicate that the attacks could not identify whether members of the training data were distinguishable from non-members based on the model output. This indicates that the model does not provide any strong evidence into the identification of the individuals in the training data set and there is not yet empirical evidence it is unsafe to distribute the model for general use.
Reinforcement Learning in Healthcare: A Survey
Aug 22, 2019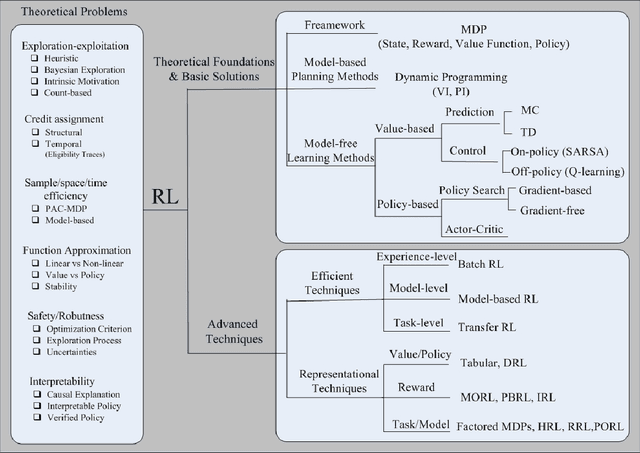
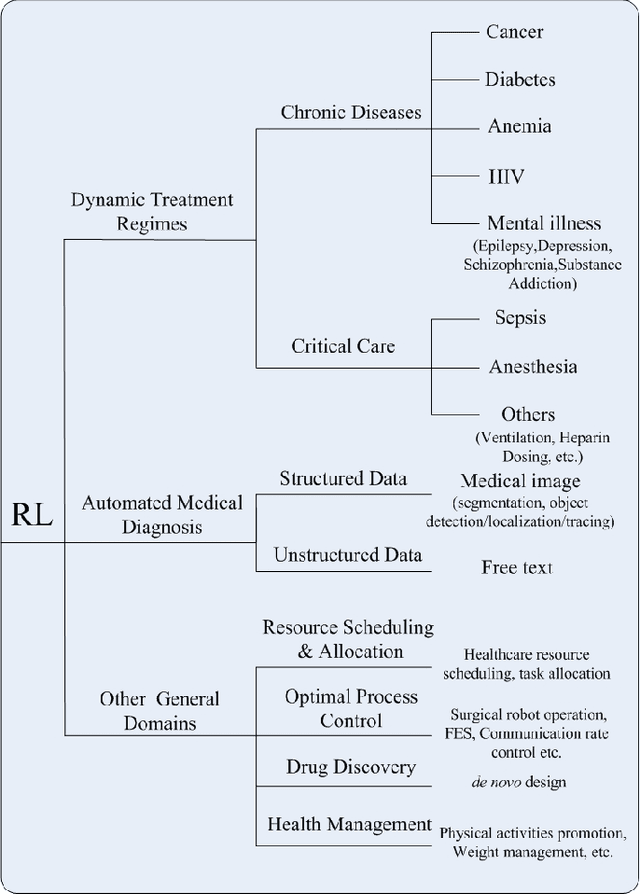
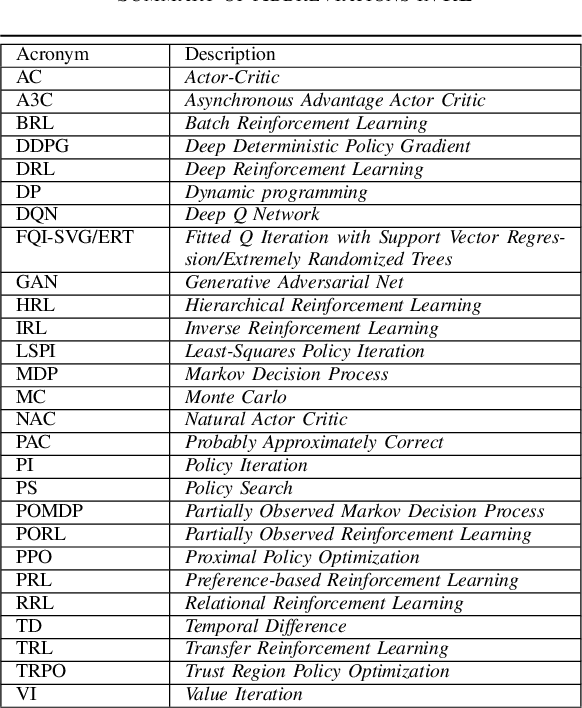
Abstract:As a subfield of machine learning, \emph{reinforcement learning} (RL) aims at empowering one's capabilities in behavioural decision making by using interaction experience with the world and an evaluative feedback. Unlike traditional supervised learning methods that usually rely on one-shot, exhaustive and supervised reward signals, RL tackles with sequential decision making problems with sampled, evaluative and delayed feedback simultaneously. Such distinctive features make RL technique a suitable candidate for developing powerful solutions in a variety of healthcare domains, where diagnosing decisions or treatment regimes are usually characterized by a prolonged and sequential procedure. This survey will discuss the broad applications of RL techniques in healthcare domains, in order to provide the research community with systematic understanding of theoretical foundations, enabling methods and techniques, existing challenges, and new insights of this emerging paradigm. By first briefly examining theoretical foundations and key techniques in RL research from efficient and representational directions, we then provide an overview of RL applications in a variety of healthcare domains, ranging from dynamic treatment regimes in chronic diseases and critical care, automated medical diagnosis from both unstructured and structured clinical data, as well as many other control or scheduling domains that have infiltrated many aspects of a healthcare system. Finally, we summarize the challenges and open issues in current research, and point out some potential solutions and directions for future research.
DeepAISE -- An End-to-End Development and Deployment of a Recurrent Neural Survival Model for Early Prediction of Sepsis
Aug 10, 2019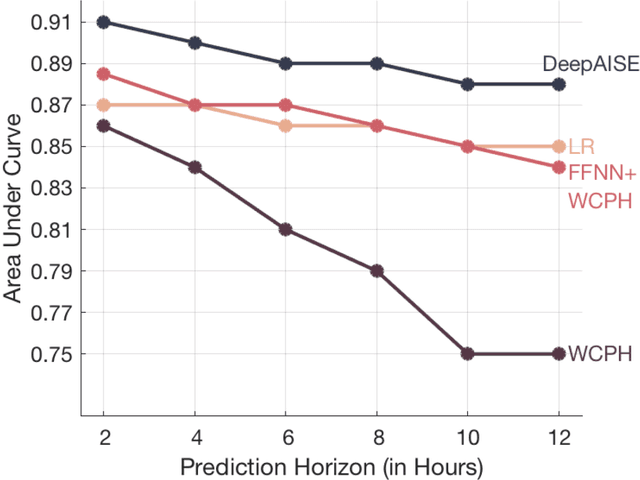
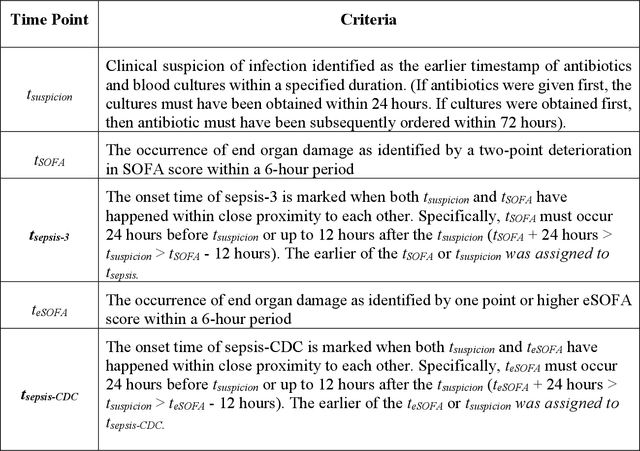
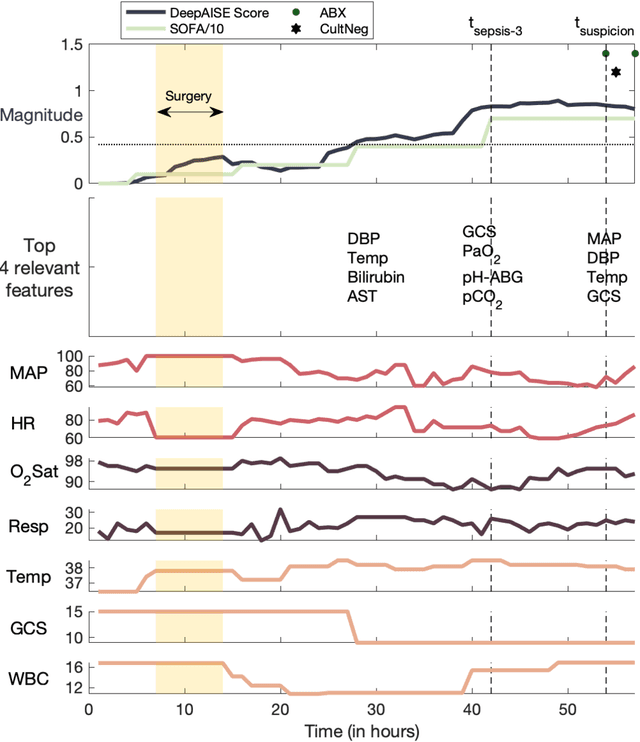
Abstract:Sepsis, a dysregulated immune system response to infection, is among the leading causes of morbidity, mortality, and cost overruns in the Intensive Care Unit (ICU). Early prediction of sepsis can improve situational awareness amongst clinicians and facilitate timely, protective interventions. While the application of predictive analytics in ICU patients has shown early promising results, much of the work has been encumbered by high false-alarm rates. Efforts to improve specificity have been limited by several factors, most notably the difficulty of labeling sepsis onset time and the low prevalence of septic-events in the ICU. Here, we present DeepAISE (Deep Artificial Intelligence Sepsis Expert), a recurrent neural survival model for the early prediction of sepsis. We show that by coupling a clinical criterion for defining sepsis onset time with a treatment policy (e.g., initiation of antibiotics within one hour of meeting the criterion), one may rank the relative utility of various criteria through offline policy evaluation. Given the optimal criterion, DeepAISE automatically learns predictive features related to higher-order interactions and temporal patterns among clinical risk factors that maximize the data likelihood of observed time to septic events. DeepAISE has been incorporated into a clinical workflow, which provides real-time hourly sepsis risk scores. A comparative study of four baseline models indicates that DeepAISE produces the most accurate predictions (AUC=0.90 and 0.87) and the lowest false alarm rates (FAR=0.20 and 0.26) in two separate cohorts (internal and external, respectively), while simultaneously producing interpretable representations of the clinical time series and risk factors.
Does the "Artificial Intelligence Clinician" learn optimal treatment strategies for sepsis in intensive care?
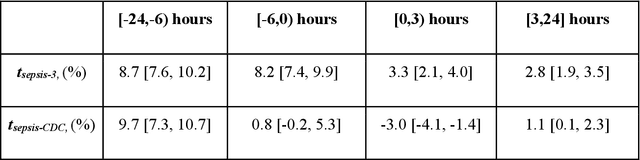
Feb 08, 2019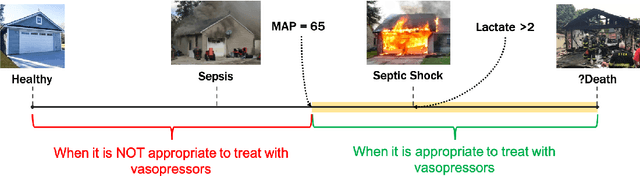
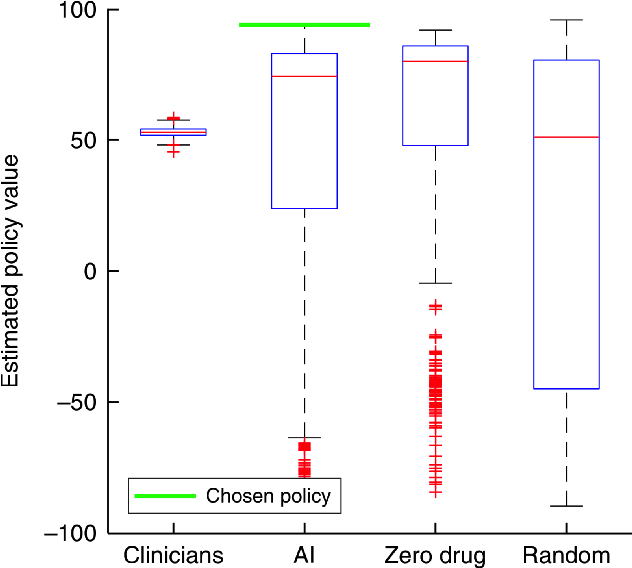
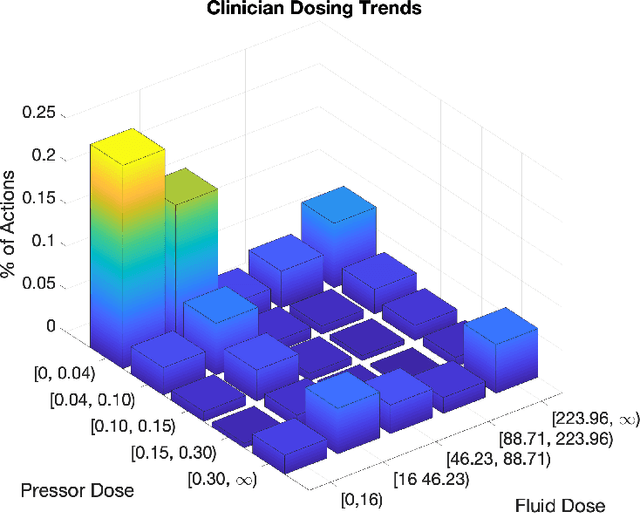
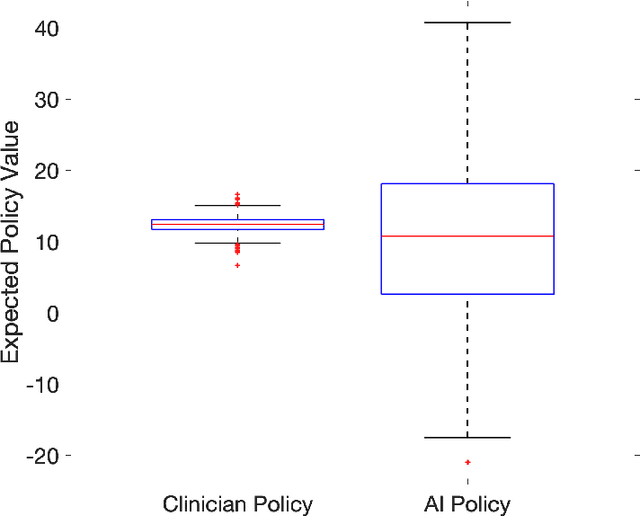
Abstract:From 2017 to 2018 the number of scientific publications found via PubMed search using the keyword "Machine Learning" increased by 46% (4,317 to 6,307). The results of studies involving machine learning, artificial intelligence (AI), and big data have captured the attention of healthcare practitioners, healthcare managers, and the public at a time when Western medicine grapples with unmitigated cost increases and public demands for accountability. The complexity involved in healthcare applications of machine learning and the size of the associated data sets has afforded many researchers an uncontested opportunity to satisfy these demands with relatively little oversight. In a recent Nature Medicine article, "The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care," Komorowski and his coauthors propose methods to train an artificial intelligence clinician to treat sepsis patients with vasopressors and IV fluids. In this post, we will closely examine the claims laid out in this paper. In particular, we will study the individual treatment profiles suggested by their AI Clinician to gain insight into how their AI Clinician intends to treat patients on an individual level.
Addressing Class Imbalance in Classification Problems of Noisy Signals by using Fourier Transform Surrogates
Jun 20, 2018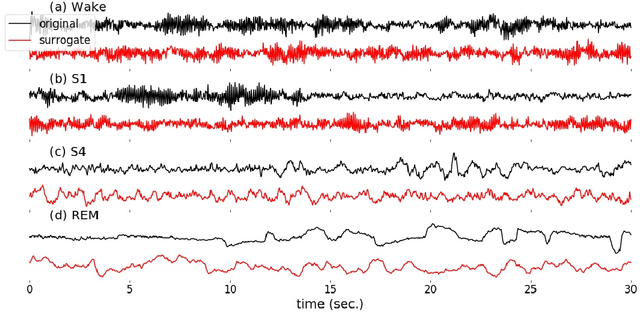
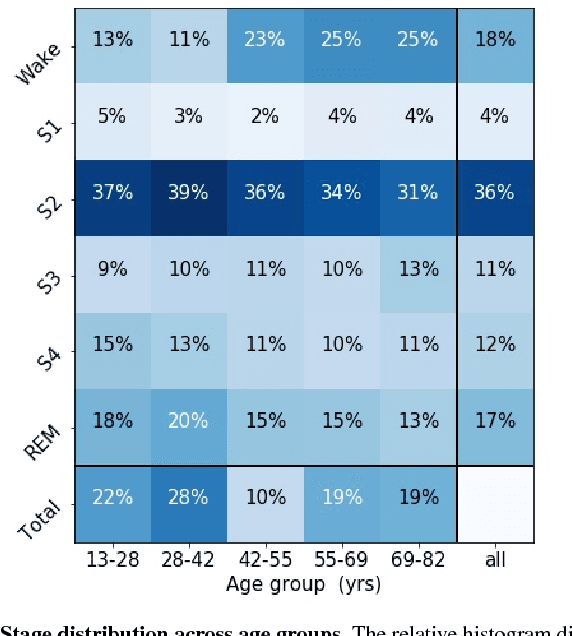
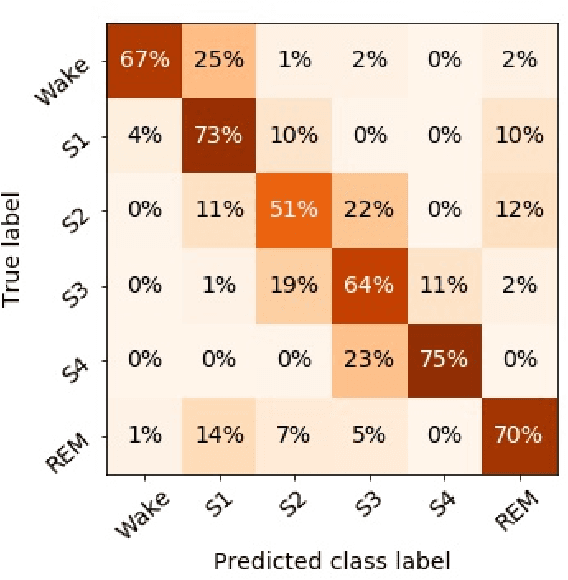
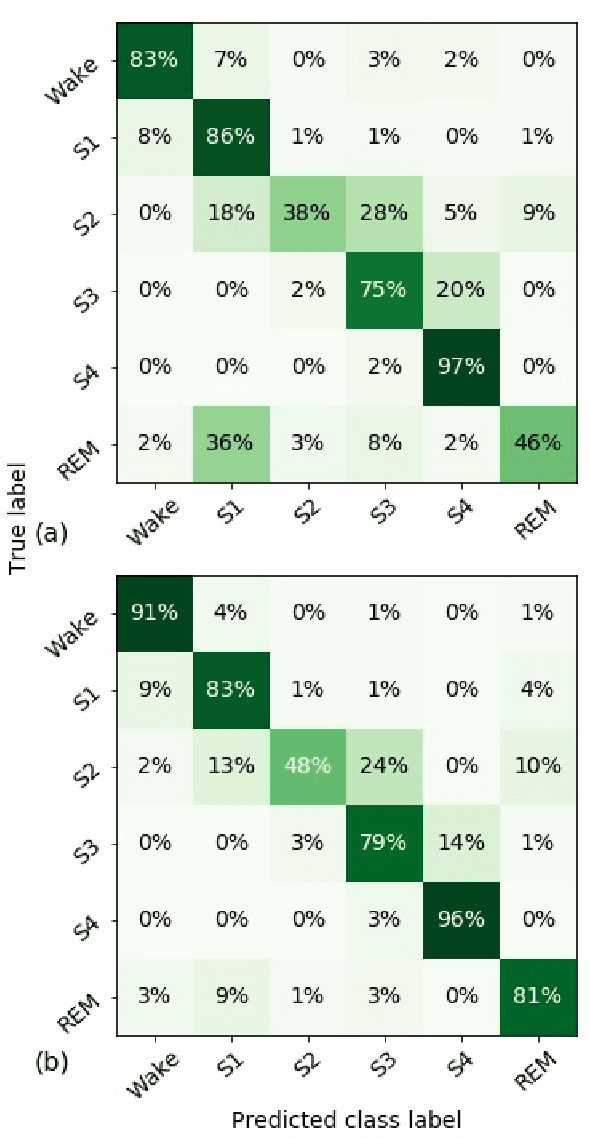
Abstract:Randomizing the Fourier-transform (FT) phases of temporal-spatial data generates surrogates that approximate examples from the data-generating distribution. We propose such FT surrogates as a novel tool to augment and analyze training of neural networks and explore the approach in the example of sleep-stage classification. By computing FT surrogates of raw EEG, EOG, and EMG signals of under-represented sleep stages, we balanced the CAPSLPDB sleep database. We then trained and tested a convolutional neural network for sleep stage classification, and found that our surrogate-based augmentation improved the mean F1-score by 7%. As another application of FT surrogates, we formulated an approach to compute saliency maps for individual sleep epochs. The visualization is based on the response of inferred class probabilities under replacement of short data segments by partial surrogates. To quantify how well the distributions of the surrogates and the original data match, we evaluated a trained classifier on surrogates of correctly classified examples, and summarized these conditional predictions in a confusion matrix. We show how such conditional confusion matrices can qualitatively explain the performance of surrogates in class balancing. The FT-surrogate augmentation approach may improve classification on noisy signals if carefully adapted to the data distribution under analysis.
Detection of Paroxysmal Atrial Fibrillation using Attention-based Bidirectional Recurrent Neural Networks
May 07, 2018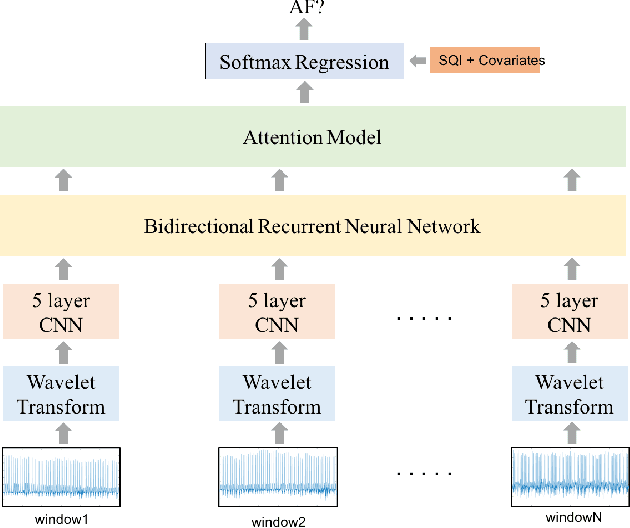
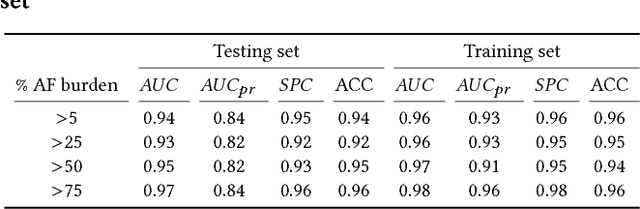
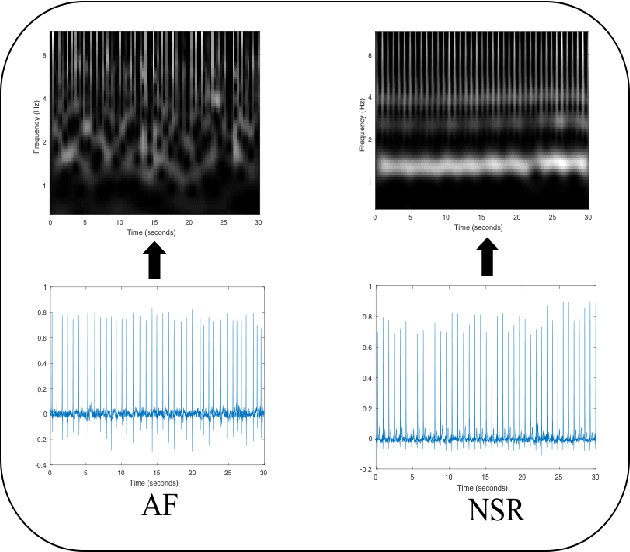
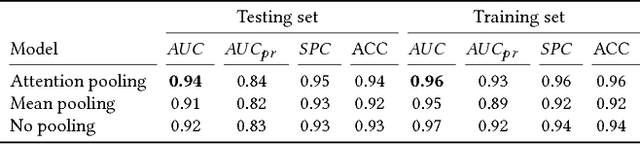
Abstract:Detection of atrial fibrillation (AF), a type of cardiac arrhythmia, is difficult since many cases of AF are usually clinically silent and undiagnosed. In particular paroxysmal AF is a form of AF that occurs occasionally, and has a higher probability of being undetected. In this work, we present an attention based deep learning framework for detection of paroxysmal AF episodes from a sequence of windows. Time-frequency representation of 30 seconds recording windows, over a 10 minute data segment, are fed sequentially into a deep convolutional neural network for image-based feature extraction, which are then presented to a bidirectional recurrent neural network with an attention layer for AF detection. To demonstrate the effectiveness of the proposed framework for transient AF detection, we use a database of 24 hour Holter Electrocardiogram (ECG) recordings acquired from 2850 patients at the University of Virginia heart station. The algorithm achieves an AUC of 0.94 on the testing set, which exceeds the performance of baseline models. We also demonstrate the cross-domain generalizablity of the approach by adapting the learned model parameters from one recording modality (ECG) to another (photoplethysmogram) with improved AF detection performance. The proposed high accuracy, low false alarm algorithm for detecting paroxysmal AF has potential applications in long-term monitoring using wearable sensors.
Patient Flow Prediction via Discriminative Learning of Mutually-Correcting Processes
Nov 10, 2016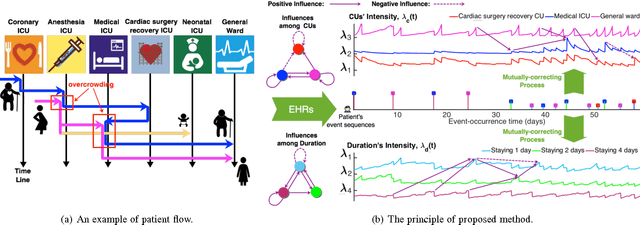
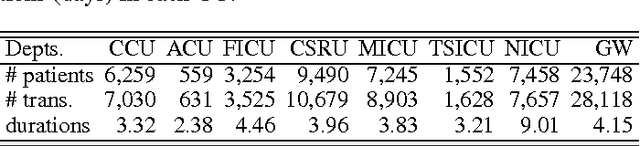
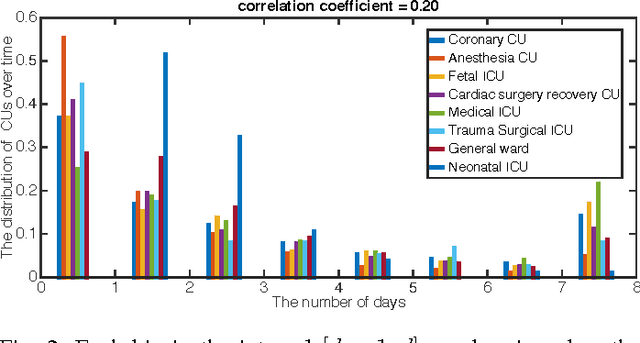

Abstract:Over the past decade the rate of care unit (CU) use in the United States has been increasing. With an aging population and ever-growing demand for medical care, effective management of patients' transitions among different care facilities will prove indispensible for shortening the length of hospital stays, improving patient outcomes, allocating critical care resources, and reducing preventable re-admissions. In this paper, we focus on an important problem of predicting the so-called "patient flow" from longitudinal electronic health records (EHRs), which has not been explored via existing machine learning techniques. By treating a sequence of transition events as a point process, we develop a novel framework for modeling patient flow through various CUs and jointly predicting patients' destination CUs and duration days. Instead of learning a generative point process model via maximum likelihood estimation, we propose a novel discriminative learning algorithm aiming at improving the prediction of transition events in the case of sparse data. By parameterizing the proposed model as a mutually-correcting process, we formulate the estimation problem via generalized linear models, which lends itself to efficient learning based on alternating direction method of multipliers (ADMM). Furthermore, we achieve simultaneous feature selection and learning by adding a group-lasso regularizer to the ADMM algorithm. Additionally, for suppressing the negative influence of data imbalance on the learning of model, we synthesize auxiliary training data for the classes with extremely few samples, and improve the robustness of our learning method accordingly. Testing on real-world data, we show that our method obtains superior performance in terms of accuracy of predicting the destination CU transition and duration of each CU occupancy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge