Mauricio Orbes-Arteaga
MAIS: Memory-Attention for Interactive Segmentation
May 12, 2025



Abstract:Interactive medical segmentation reduces annotation effort by refining predictions through user feedback. Vision Transformer (ViT)-based models, such as the Segment Anything Model (SAM), achieve state-of-the-art performance using user clicks and prior masks as prompts. However, existing methods treat interactions as independent events, leading to redundant corrections and limited refinement gains. We address this by introducing MAIS, a Memory-Attention mechanism for Interactive Segmentation that stores past user inputs and segmentation states, enabling temporal context integration. Our approach enhances ViT-based segmentation across diverse imaging modalities, achieving more efficient and accurate refinements.
Augmentation based unsupervised domain adaptation
Feb 23, 2022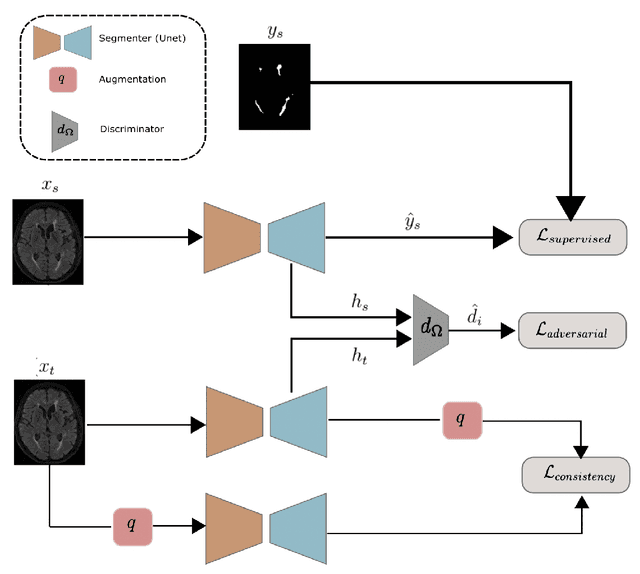
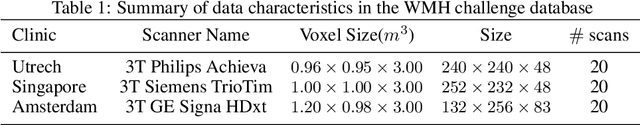
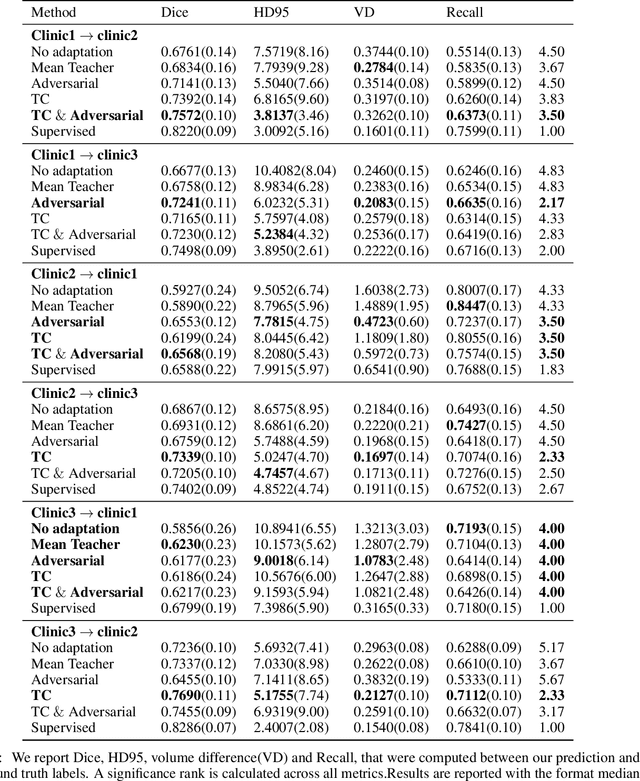
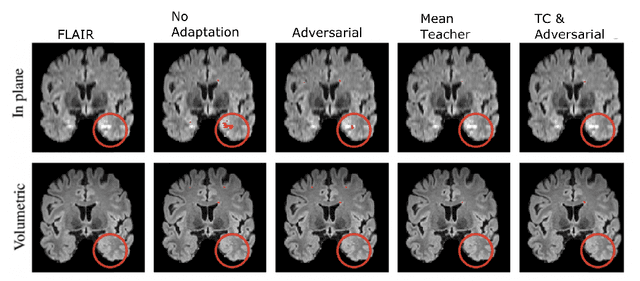
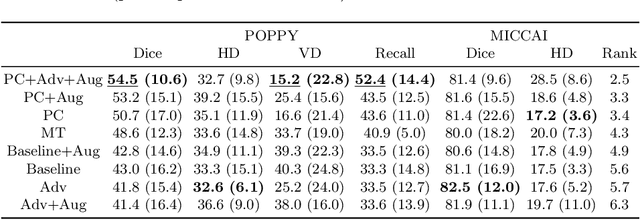
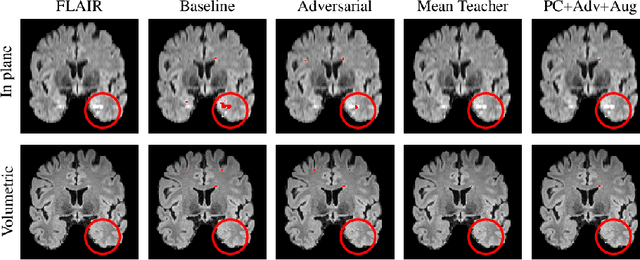
Abstract:The insertion of deep learning in medical image analysis had lead to the development of state-of-the art strategies in several applications such a disease classification, as well as abnormality detection and segmentation. However, even the most advanced methods require a huge and diverse amount of data to generalize. Because in realistic clinical scenarios, data acquisition and annotation is expensive, deep learning models trained on small and unrepresentative data tend to outperform when deployed in data that differs from the one used for training (e.g data from different scanners). In this work, we proposed a domain adaptation methodology to alleviate this problem in segmentation models. Our approach takes advantage of the properties of adversarial domain adaptation and consistency training to achieve more robust adaptation. Using two datasets with white matter hyperintensities (WMH) annotations, we demonstrated that the proposed method improves model generalization even in corner cases where individual strategies tend to fail.
DermX: an end-to-end framework for explainable automated dermatological diagnosis
Feb 14, 2022


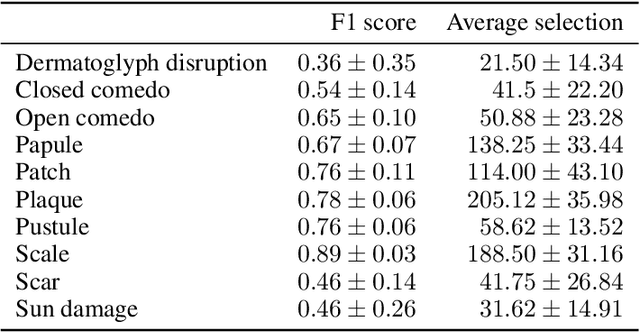
Abstract:Dermatological diagnosis automation is essential in addressing the high prevalence of skin diseases and critical shortage of dermatologists. Despite approaching expert-level diagnosis performance, convolutional neural network (ConvNet) adoption in clinical practice is impeded by their limited explainability, and by subjective, expensive explainability validations. We introduce DermX and DermX+, an end-to-end framework for explainable automated dermatological diagnosis. DermX is a clinically-inspired explainable dermatological diagnosis ConvNet, trained using DermXDB, a 554 images dataset annotated by eight dermatologists with diagnoses and supporting explanations. DermX+ extends DermX with guided attention training for explanation attention maps. Both methods achieve near-expert diagnosis performance, with DermX, DermX+, and dermatologist F1 scores of 0.79, 0.79, and 0.87, respectively. We assess the explanation plausibility in terms of identification and localization, by comparing model-selected with dermatologist-selected explanations, and gradient-weighted class-activation maps with dermatologist explanation maps. Both DermX and DermX+ obtain an identification F1 score of 0.78. The localization F1 score is 0.39 for DermX and 0.35 for DermX+. Explanation faithfulness is assessed through contrasting samples, DermX obtaining 0.53 faithfulness and DermX+ 0.25. These results show that explainability does not necessarily come at the expense of predictive power, as our high-performance models provide both plausible and faithful explanations for their diagnoses.
Test-time Unsupervised Domain Adaptation
Oct 05, 2020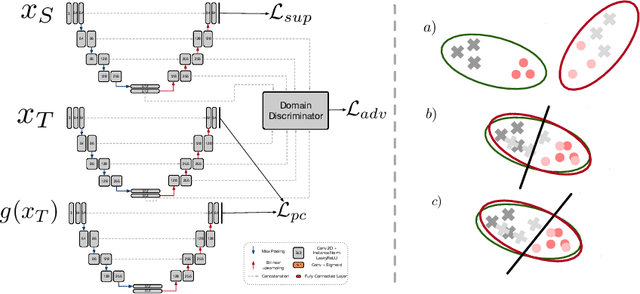
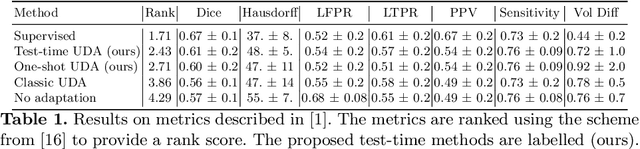
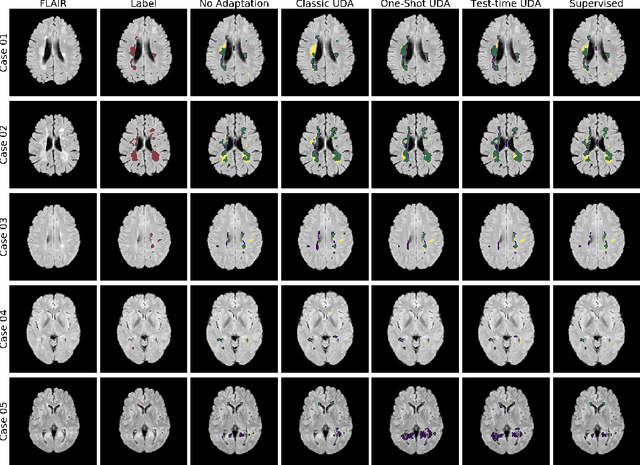
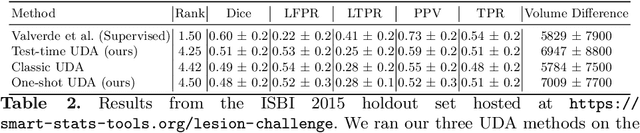
Abstract:Convolutional neural networks trained on publicly available medical imaging datasets (source domain) rarely generalise to different scanners or acquisition protocols (target domain). This motivates the active field of domain adaptation. While some approaches to the problem require labeled data from the target domain, others adopt an unsupervised approach to domain adaptation (UDA). Evaluating UDA methods consists of measuring the model's ability to generalise to unseen data in the target domain. In this work, we argue that this is not as useful as adapting to the test set directly. We therefore propose an evaluation framework where we perform test-time UDA on each subject separately. We show that models adapted to a specific target subject from the target domain outperform a domain adaptation method which has seen more data of the target domain but not this specific target subject. This result supports the thesis that unsupervised domain adaptation should be used at test-time, even if only using a single target-domain subject
Multi-Domain Adaptation in Brain MRI through Paired Consistency and Adversarial Learning
Sep 17, 2019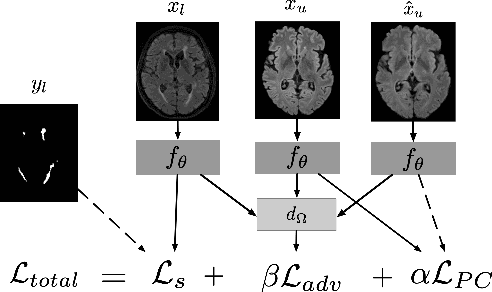
Abstract:Supervised learning algorithms trained on medical images will often fail to generalize across changes in acquisition parameters. Recent work in domain adaptation addresses this challenge and successfully leverages labeled data in a source domain to perform well on an unlabeled target domain. Inspired by recent work in semi-supervised learning we introduce a novel method to adapt from one source domain to $n$ target domains (as long as there is paired data covering all domains). Our multi-domain adaptation method utilises a consistency loss combined with adversarial learning. We provide results on white matter lesion hyperintensity segmentation from brain MRIs using the MICCAI 2017 challenge data as the source domain and two target domains. The proposed method significantly outperforms other domain adaptation baselines.
Knowledge distillation for semi-supervised domain adaptation
Aug 16, 2019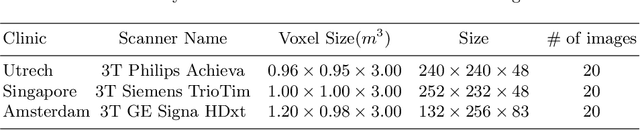
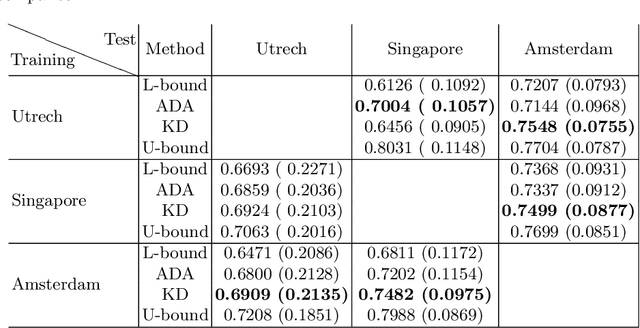
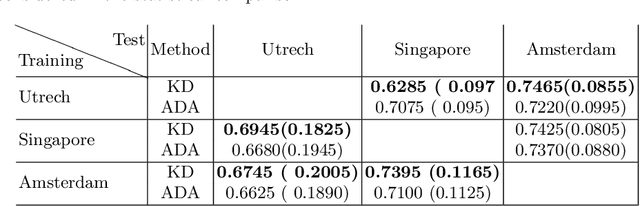
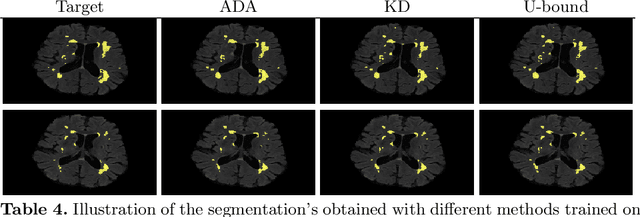
Abstract:In the absence of sufficient data variation (e.g., scanner and protocol variability) in annotated data, deep neural networks (DNNs) tend to overfit during training. As a result, their performance is significantly lower on data from unseen sources compared to the performance on data from the same source as the training data. Semi-supervised domain adaptation methods can alleviate this problem by tuning networks to new target domains without the need for annotated data from these domains. Adversarial domain adaptation (ADA) methods are a popular choice that aim to train networks in such a way that the features generated are domain agnostic. However, these methods require careful dataset-specific selection of hyperparameters such as the complexity of the discriminator in order to achieve a reasonable performance. We propose to use knowledge distillation (KD) -- an efficient way of transferring knowledge between different DNNs -- for semi-supervised domain adaption of DNNs. It does not require dataset-specific hyperparameter tuning, making it generally applicable. The proposed method is compared to ADA for segmentation of white matter hyperintensities (WMH) in magnetic resonance imaging (MRI) scans generated by scanners that are not a part of the training set. Compared with both the baseline DNN (trained on source domain only and without any adaption to target domain) and with using ADA for semi-supervised domain adaptation, the proposed method achieves significantly higher WMH dice scores.
Simultaneous synthesis of FLAIR and segmentation of white matter hypointensities from T1 MRIs
Aug 20, 2018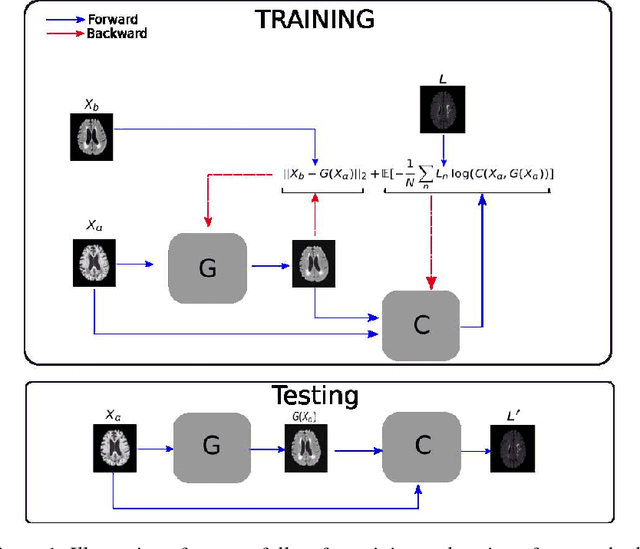
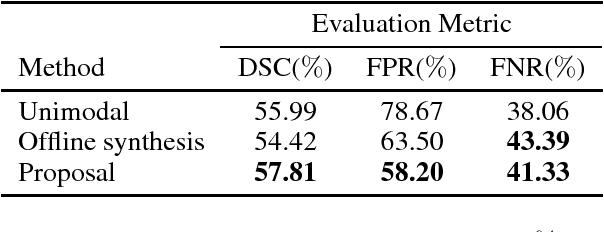
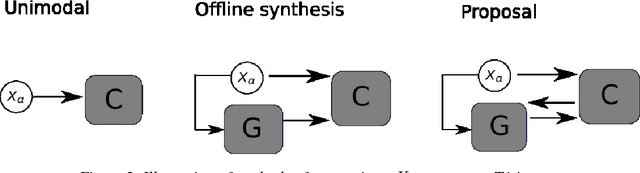
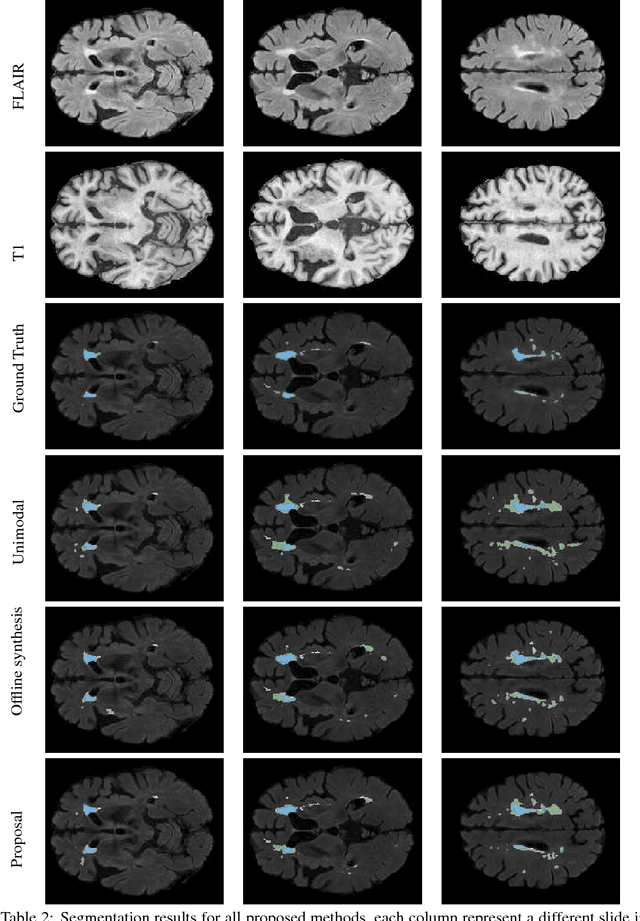
Abstract:Segmenting vascular pathologies such as white matter lesions in Brain magnetic resonance images (MRIs) require acquisition of multiple sequences such as T1-weighted (T1-w) --on which lesions appear hypointense-- and fluid attenuated inversion recovery (FLAIR) sequence --where lesions appear hyperintense--. However, most of the existing retrospective datasets do not consist of FLAIR sequences. Existing missing modality imputation methods separate the process of imputation, and the process of segmentation. In this paper, we propose a method to link both modality imputation and segmentation using convolutional neural networks. We show that by jointly optimizing the imputation network and the segmentation network, the method not only produces more realistic synthetic FLAIR images from T1-w images, but also improves the segmentation of WMH from T1-w images only.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge