Kee Yuan Ngiam
AI-generated data contamination erodes pathological variability and diagnostic reliability
Jan 21, 2026Abstract:Generative artificial intelligence (AI) is rapidly populating medical records with synthetic content, creating a feedback loop where future models are increasingly at risk of training on uncurated AI-generated data. However, the clinical consequences of this AI-generated data contamination remain unexplored. Here, we show that in the absence of mandatory human verification, this self-referential cycle drives a rapid erosion of pathological variability and diagnostic reliability. By analysing more than 800,000 synthetic data points across clinical text generation, vision-language reporting, and medical image synthesis, we find that models progressively converge toward generic phenotypes regardless of the model architecture. Specifically, rare but critical findings, including pneumothorax and effusions, vanish from the synthetic content generated by AI models, while demographic representations skew heavily toward middle-aged male phenotypes. Crucially, this degradation is masked by false diagnostic confidence; models continue to issue reassuring reports while failing to detect life-threatening pathology, with false reassurance rates tripling to 40%. Blinded physician evaluation confirms that this decoupling of confidence and accuracy renders AI-generated documentation clinically useless after just two generations. We systematically evaluate three mitigation strategies, finding that while synthetic volume scaling fails to prevent collapse, mixing real data with quality-aware filtering effectively preserves diversity. Ultimately, our results suggest that without policy-mandated human oversight, the deployment of generative AI threatens to degrade the very healthcare data ecosystems it relies upon.
HoloPOCUS: Portable Mixed-Reality 3D Ultrasound Tracking, Reconstruction and Overlay
Aug 26, 2023Abstract:Ultrasound (US) imaging provides a safe and accessible solution to procedural guidance and diagnostic imaging. The effective usage of conventional 2D US for interventional guidance requires extensive experience to project the image plane onto the patient, and the interpretation of images in diagnostics suffers from high intra- and inter-user variability. 3D US reconstruction allows for more consistent diagnosis and interpretation, but existing solutions are limited in terms of equipment and applicability in real-time navigation. To address these issues, we propose HoloPOCUS - a mixed reality US system (MR-US) that overlays rich US information onto the user's vision in a point-of-care setting. HoloPOCUS extends existing MR-US methods beyond placing a US plane in the user's vision to include a 3D reconstruction and projection that can aid in procedural guidance using conventional probes. We validated a tracking pipeline that demonstrates higher accuracy compared to existing MR-US works. Furthermore, user studies conducted via a phantom task showed significant improvements in navigation duration when using our proposed methods.
MLCask: Efficient Management of Component Evolution in Collaborative Data Analytics Pipelines
Oct 17, 2020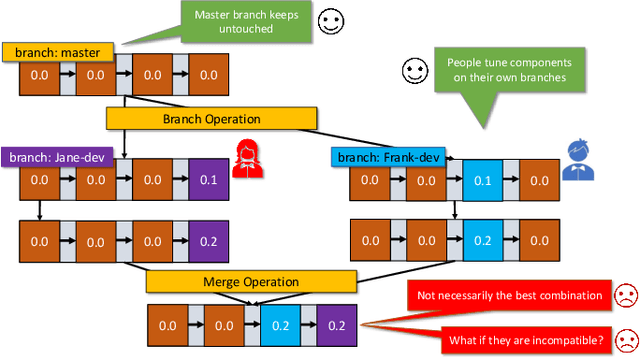
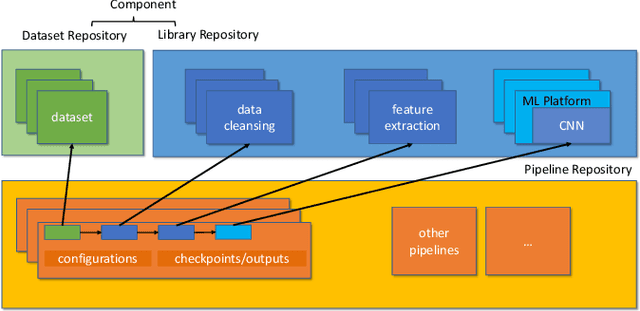
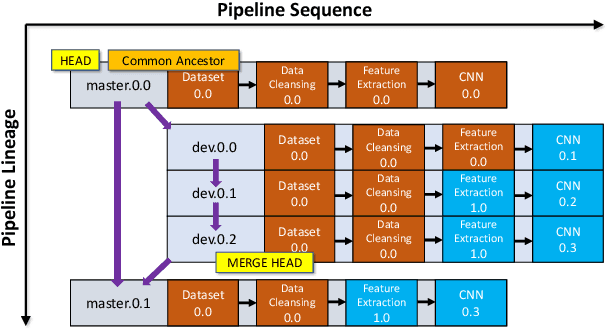
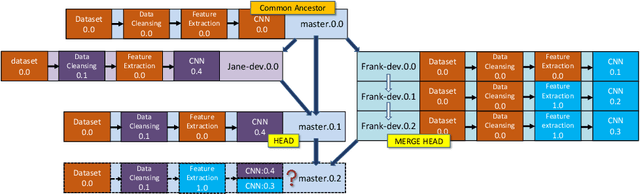
Abstract:With the ever-increasing adoption of machine learning for data analytics, maintaining a machine learning pipeline is becoming more complex as both the datasets and trained models evolve with time. In a collaborative environment, the changes and updates due to pipeline evolution often cause cumbersome coordination and maintenance work, raising the costs and making it hard to use. Existing solutions, unfortunately, do not address the version evolution problem, especially in a collaborative environment where non-linear version control semantics are necessary to isolate operations made by different user roles. The lack of version control semantics also incurs unnecessary storage consumption and lowers efficiency due to data duplication and repeated data pre-processing, which are avoidable. In this paper, we identify two main challenges that arise during the deployment of machine learning pipelines, and address them with the design of versioning for an end-to-end analytics system MLCask. The system supports multiple user roles with the ability to perform Git-like branching and merging operations in the context of the machine learning pipelines. We define and accelerate the metric-driven merge operation by pruning the pipeline search tree using reusable history records and pipeline compatibility information. Further, we design and implement the prioritized pipeline search, which gives preference to the pipelines that probably yield better performance. The effectiveness of MLCask is evaluated through an extensive study over several real-world deployment cases. The performance evaluation shows that the proposed merge operation is up to 7.8x faster and saves up to 11.9x storage space than the baseline method that does not utilize history records.
TRACER: A Framework for Facilitating Accurate and Interpretable Analytics for High Stakes Applications
Mar 24, 2020


Abstract:In high stakes applications such as healthcare and finance analytics, the interpretability of predictive models is required and necessary for domain practitioners to trust the predictions. Traditional machine learning models, e.g., logistic regression (LR), are easy to interpret in nature. However, many of these models aggregate time-series data without considering the temporal correlations and variations. Therefore, their performance cannot match up to recurrent neural network (RNN) based models, which are nonetheless difficult to interpret. In this paper, we propose a general framework TRACER to facilitate accurate and interpretable predictions, with a novel model TITV devised for healthcare analytics and other high stakes applications such as financial investment and risk management. Different from LR and other existing RNN-based models, TITV is designed to capture both the time-invariant and the time-variant feature importance using a feature-wise transformation subnetwork and a self-attention subnetwork, for the feature influence shared over the entire time series and the time-related importance respectively. Healthcare analytics is adopted as a driving use case, and we note that the proposed TRACER is also applicable to other domains, e.g., fintech. We evaluate the accuracy of TRACER extensively in two real-world hospital datasets, and our doctors/clinicians further validate the interpretability of TRACER in both the patient level and the feature level. Besides, TRACER is also validated in a high stakes financial application and a critical temperature forecasting application. The experimental results confirm that TRACER facilitates both accurate and interpretable analytics for high stakes applications.
Deep Reinforcement Learning for Clinical Decision Support: A Brief Survey
Jul 22, 2019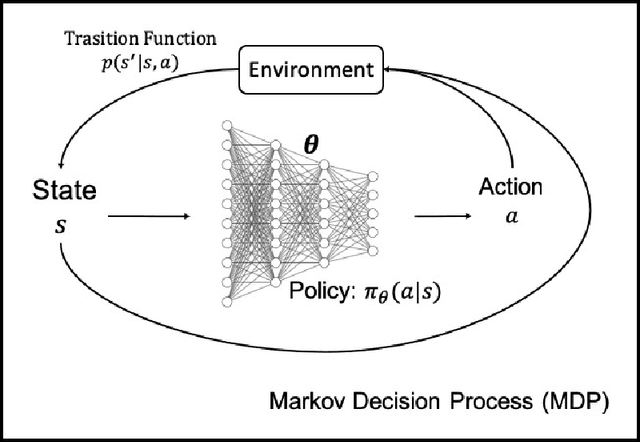

Abstract:Owe to the recent advancements in Artificial Intelligence especially deep learning, many data-driven decision support systems have been implemented to facilitate medical doctors in delivering personalized care. We focus on the deep reinforcement learning (DRL) models in this paper. DRL models have demonstrated human-level or even superior performance in the tasks of computer vision and game playings, such as Go and Atari game. However, the adoption of deep reinforcement learning techniques in clinical decision optimization is still rare. We present the first survey that summarizes reinforcement learning algorithms with Deep Neural Networks (DNN) on clinical decision support. We also discuss some case studies, where different DRL algorithms were applied to address various clinical challenges. We further compare and contrast the advantages and limitations of various DRL algorithms and present a preliminary guide on how to choose the appropriate DRL algorithm for particular clinical applications.
A Self-Correcting Deep Learning Approach to Predict Acute Conditions in Critical Care
Jan 14, 2019
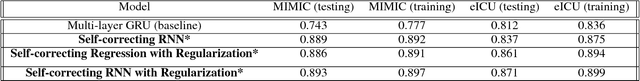
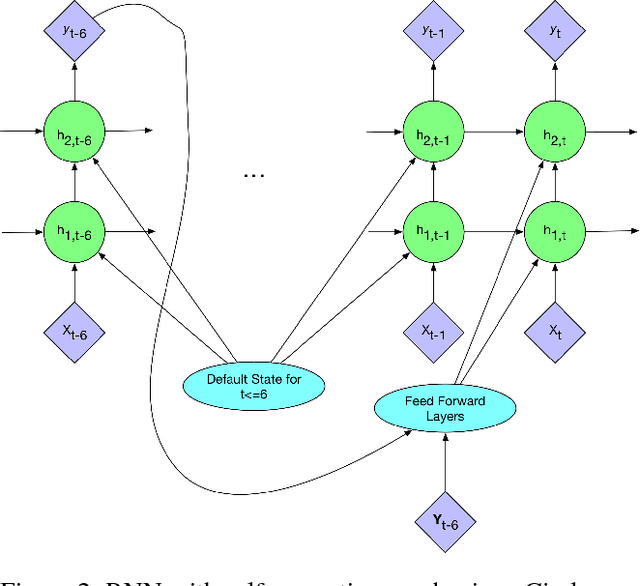
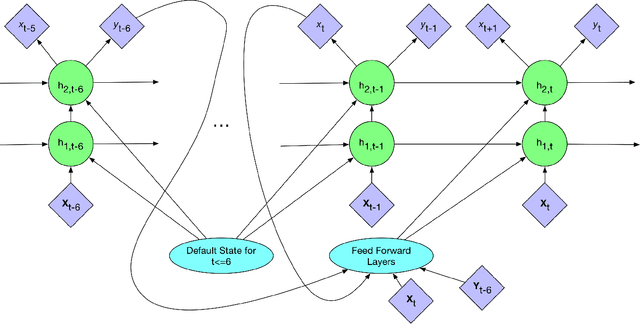
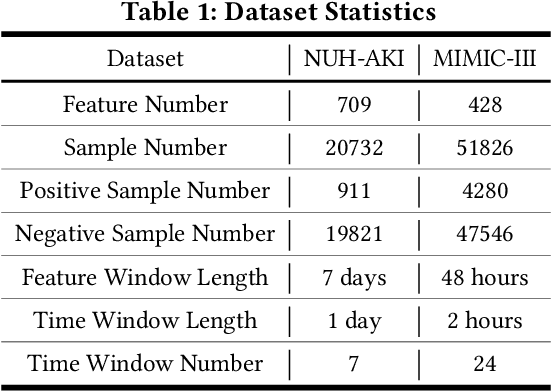
Abstract:In critical care, intensivists are required to continuously monitor high dimensional vital signs and lab measurements to detect and diagnose acute patient conditions. This has always been a challenging task. In this study, we propose a novel self-correcting deep learning prediction approach to address this challenge. We focus on an example of the prediction of acute kidney injury (AKI). Compared with the existing models, our method has a number of distinct features: we utilized the accumulative data of patients in ICU; we developed a self-correcting mechanism that feeds errors from the previous predictions back into the network; we also proposed a regularization method that takes into account not only the model's prediction error on the label but also its estimation errors on the input data. This mechanism is applied in both regression and classification tasks. We compared the performance of our proposed method with the conventional deep learning models on two real-world clinical datasets and demonstrated that our proposed model constantly outperforms these baseline models. In particular, the proposed model achieved area under ROC curve at 0.893 on the MIMIC III dataset, and 0.871 on the Philips eICU dataset.
Medical Concept Embedding with Time-Aware Attention
Jun 06, 2018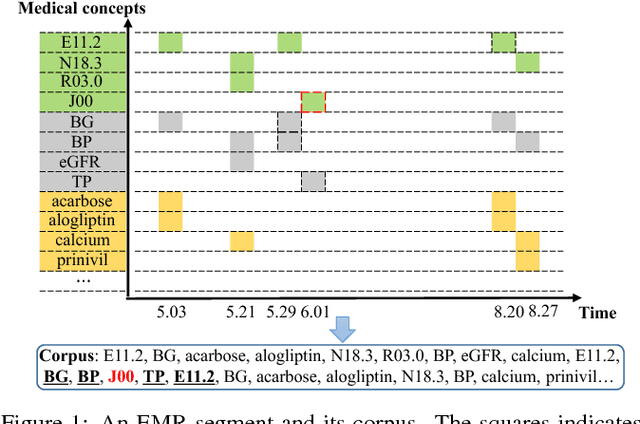
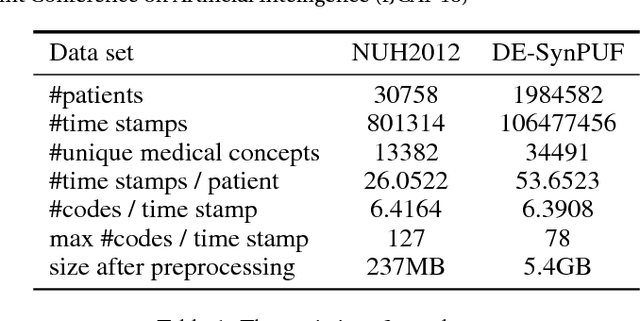
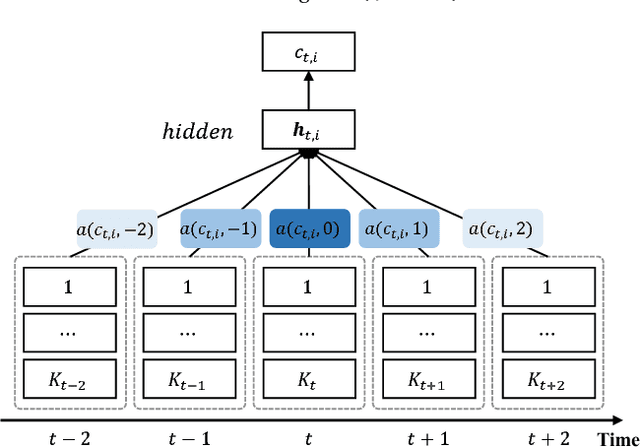
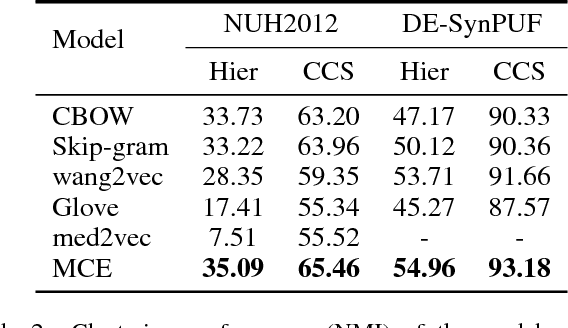
Abstract:Embeddings of medical concepts such as medication, procedure and diagnosis codes in Electronic Medical Records (EMRs) are central to healthcare analytics. Previous work on medical concept embedding takes medical concepts and EMRs as words and documents respectively. Nevertheless, such models miss out the temporal nature of EMR data. On the one hand, two consecutive medical concepts do not indicate they are temporally close, but the correlations between them can be revealed by the time gap. On the other hand, the temporal scopes of medical concepts often vary greatly (e.g., \textit{common cold} and \textit{diabetes}). In this paper, we propose to incorporate the temporal information to embed medical codes. Based on the Continuous Bag-of-Words model, we employ the attention mechanism to learn a "soft" time-aware context window for each medical concept. Experiments on public and proprietary datasets through clustering and nearest neighbour search tasks demonstrate the effectiveness of our model, showing that it outperforms five state-of-the-art baselines.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge