Joseph R. Ledsam
AVIDa-hIL6: A Large-Scale VHH Dataset Produced from an Immunized Alpaca for Predicting Antigen-Antibody Interactions
Jun 06, 2023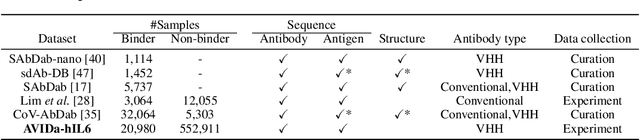
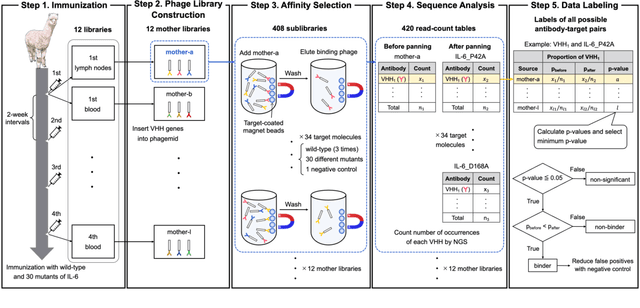
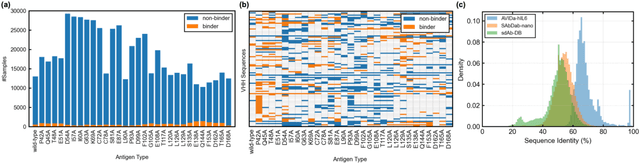

Abstract:Antibodies have become an important class of therapeutic agents to treat human diseases. To accelerate therapeutic antibody discovery, computational methods, especially machine learning, have attracted considerable interest for predicting specific interactions between antibody candidates and target antigens such as viruses and bacteria. However, the publicly available datasets in existing works have notable limitations, such as small sizes and the lack of non-binding samples and exact amino acid sequences. To overcome these limitations, we have developed AVIDa-hIL6, a large-scale dataset for predicting antigen-antibody interactions in the variable domain of heavy chain of heavy chain antibodies (VHHs), produced from an alpaca immunized with the human interleukin-6 (IL-6) protein, as antigens. By leveraging the simple structure of VHHs, which facilitates identification of full-length amino acid sequences by DNA sequencing technology, AVIDa-hIL6 contains 573,891 antigen-VHH pairs with amino acid sequences. All the antigen-VHH pairs have reliable labels for binding or non-binding, as generated by a novel labeling method. Furthermore, via introduction of artificial mutations, AVIDa-hIL6 contains 30 different mutants in addition to wild-type IL-6 protein. This characteristic provides opportunities to develop machine learning models for predicting changes in antibody binding by antigen mutations. We report experimental benchmark results on AVIDa-hIL6 by using neural network-based baseline models. The results indicate that the existing models have potential, but further research is needed to generalize them to predict effective antibodies against unknown mutants. The dataset is available at https://avida-hil6.cognanous.com.
Contrastive Training for Improved Out-of-Distribution Detection
Jul 10, 2020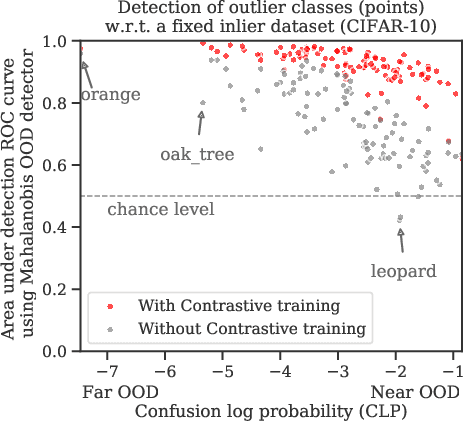
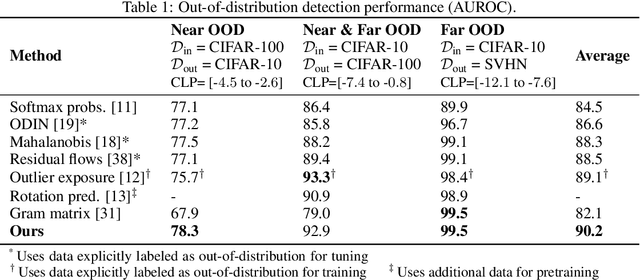
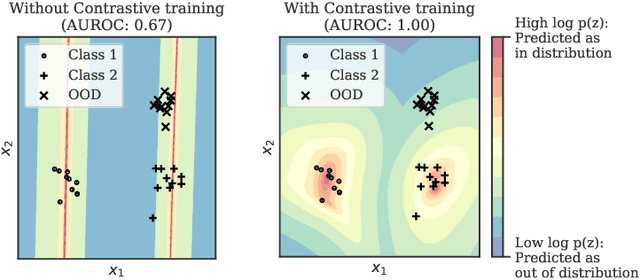
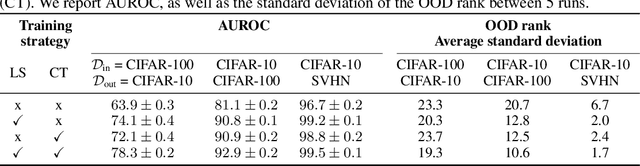
Abstract:Reliable detection of out-of-distribution (OOD) inputs is increasingly understood to be a precondition for deployment of machine learning systems. This paper proposes and investigates the use of contrastive training to boost OOD detection performance. Unlike leading methods for OOD detection, our approach does not require access to examples labeled explicitly as OOD, which can be difficult to collect in practice. We show in extensive experiments that contrastive training significantly helps OOD detection performance on a number of common benchmarks. By introducing and employing the Confusion Log Probability (CLP) score, which quantifies the difficulty of the OOD detection task by capturing the similarity of inlier and outlier datasets, we show that our method especially improves performance in the `near OOD' classes -- a particularly challenging setting for previous methods.
A Probabilistic U-Net for Segmentation of Ambiguous Images
Oct 29, 2018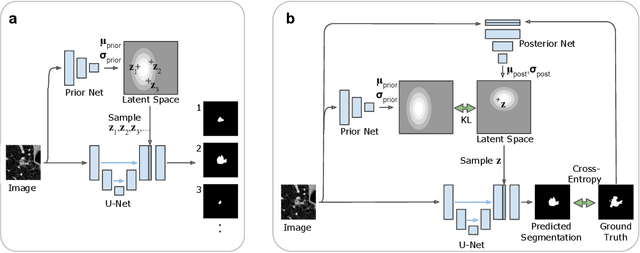

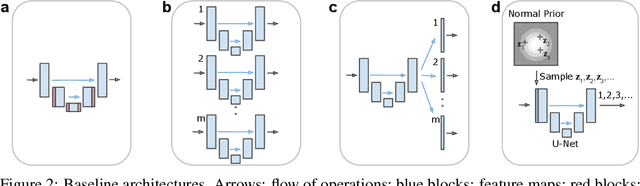

Abstract:Many real-world vision problems suffer from inherent ambiguities. In clinical applications for example, it might not be clear from a CT scan alone which particular region is cancer tissue. Therefore a group of graders typically produces a set of diverse but plausible segmentations. We consider the task of learning a distribution over segmentations given an input. To this end we propose a generative segmentation model based on a combination of a U-Net with a conditional variational autoencoder that is capable of efficiently producing an unlimited number of plausible hypotheses. We show on a lung abnormalities segmentation task and on a Cityscapes segmentation task that our model reproduces the possible segmentation variants as well as the frequencies with which they occur, doing so significantly better than published approaches. These models could have a high impact in real-world applications, such as being used as clinical decision-making algorithms accounting for multiple plausible semantic segmentation hypotheses to provide possible diagnoses and recommend further actions to resolve the present ambiguities.
Deep learning to achieve clinically applicable segmentation of head and neck anatomy for radiotherapy
Sep 12, 2018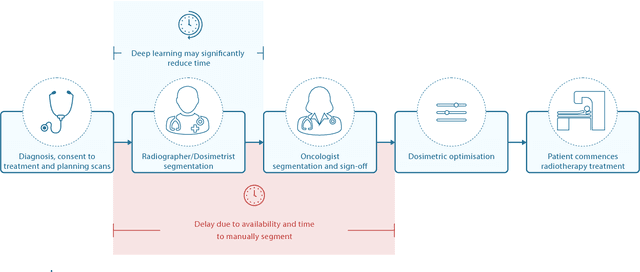
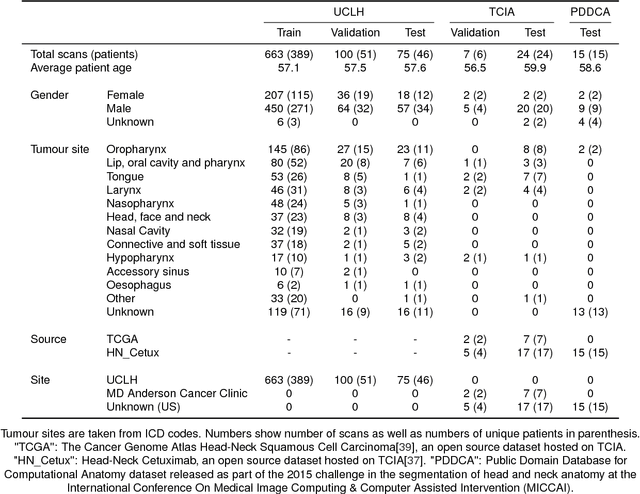
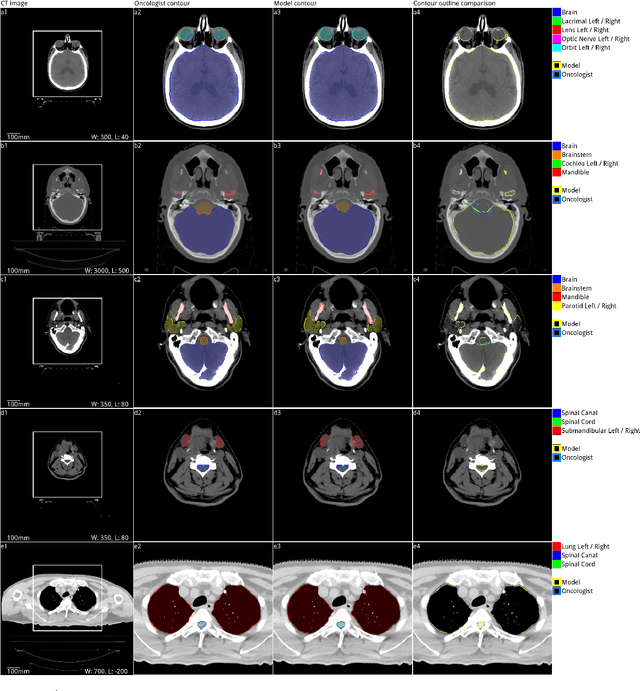
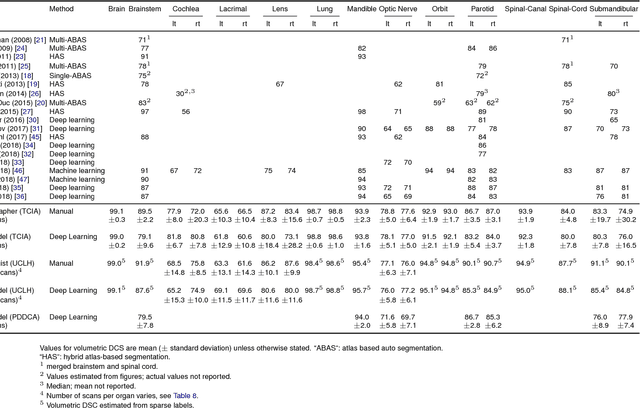
Abstract:Over half a million individuals are diagnosed with head and neck cancer each year worldwide. Radiotherapy is an important curative treatment for this disease, but it requires manually intensive delineation of radiosensitive organs at risk (OARs). This planning process can delay treatment commencement. While auto-segmentation algorithms offer a potentially time-saving solution, the challenges in defining, quantifying and achieving expert performance remain. Adopting a deep learning approach, we demonstrate a 3D U-Net architecture that achieves performance similar to experts in delineating a wide range of head and neck OARs. The model was trained on a dataset of 663 deidentified computed tomography (CT) scans acquired in routine clinical practice and segmented according to consensus OAR definitions. We demonstrate its generalisability through application to an independent test set of 24 CT scans available from The Cancer Imaging Archive collected at multiple international sites previously unseen to the model, each segmented by two independent experts and consisting of 21 OARs commonly segmented in clinical practice. With appropriate validation studies and regulatory approvals, this system could improve the effectiveness of radiotherapy pathways.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge