Jinkui Hao
ProDM: Synthetic Reality-driven Property-aware Progressive Diffusion Model for Coronary Calcium Motion Correction in Non-gated Chest CT
Dec 31, 2025Abstract:Coronary artery calcium (CAC) scoring from chest CT is a well-established tool to stratify and refine clinical cardiovascular disease risk estimation. CAC quantification relies on the accurate delineation of calcified lesions, but is oftentimes affected by artifacts introduced by cardiac and respiratory motion. ECG-gated cardiac CTs substantially reduce motion artifacts, but their use in population screening and routine imaging remains limited due to gating requirements and lack of insurance coverage. Although identification of incidental CAC from non-gated chest CT is increasingly considered for it offers an accessible and widely available alternative, this modality is limited by more severe motion artifacts. We present ProDM (Property-aware Progressive Correction Diffusion Model), a generative diffusion framework that restores motion-free calcified lesions from non-gated CTs. ProDM introduces three key components: (1) a CAC motion simulation data engine that synthesizes realistic non-gated acquisitions with diverse motion trajectories directly from cardiac-gated CTs, enabling supervised training without paired data; (2) a property-aware learning strategy incorporating calcium-specific priors through a differentiable calcium consistency loss to preserve lesion integrity; and (3) a progressive correction scheme that reduces artifacts gradually across diffusion steps to enhance stability and calcium fidelity. Experiments on real patient datasets show that ProDM significantly improves CAC scoring accuracy, spatial lesion fidelity, and risk stratification performance compared with several baselines. A reader study on real non-gated scans further confirms that ProDM suppresses motion artifacts and improves clinical usability. These findings highlight the potential of progressive, property-aware frameworks for reliable CAC quantification from routine chest CT imaging.
AMA-SAM: Adversarial Multi-Domain Alignment of Segment Anything Model for High-Fidelity Histology Nuclei Segmentation
Mar 27, 2025Abstract:Accurate segmentation of cell nuclei in histopathology images is essential for numerous biomedical research and clinical applications. However, existing cell nucleus segmentation methods only consider a single dataset (i.e., primary domain), while neglecting to leverage supplementary data from diverse sources (i.e., auxiliary domains) to reduce overfitting and enhance the performance. Although incorporating multiple datasets could alleviate overfitting, it often exacerbates performance drops caused by domain shifts. In this work, we introduce Adversarial Multi-domain Alignment of Segment Anything Model (AMA-SAM) that extends the Segment Anything Model (SAM) to overcome these obstacles through two key innovations. First, we propose a Conditional Gradient Reversal Layer (CGRL), a multi-domain alignment module that harmonizes features from diverse domains to promote domain-invariant representation learning while preserving crucial discriminative features for the primary dataset. Second, we address SAM's inherent low-resolution output by designing a High-Resolution Decoder (HR-Decoder), which directly produces fine-grained segmentation maps in order to capture intricate nuclei boundaries in high-resolution histology images. To the best of our knowledge, this is the first attempt to adapt SAM for multi-dataset learning with application to histology nuclei segmentation. We validate our method on several publicly available datasets, demonstrating consistent and significant improvements over state-of-the-art approaches.
Dual-domain Multi-path Self-supervised Diffusion Model for Accelerated MRI Reconstruction
Mar 24, 2025Abstract:Magnetic resonance imaging (MRI) is a vital diagnostic tool, but its inherently long acquisition times reduce clinical efficiency and patient comfort. Recent advancements in deep learning, particularly diffusion models, have improved accelerated MRI reconstruction. However, existing diffusion models' training often relies on fully sampled data, models incur high computational costs, and often lack uncertainty estimation, limiting their clinical applicability. To overcome these challenges, we propose a novel framework, called Dual-domain Multi-path Self-supervised Diffusion Model (DMSM), that integrates a self-supervised dual-domain diffusion model training scheme, a lightweight hybrid attention network for the reconstruction diffusion model, and a multi-path inference strategy, to enhance reconstruction accuracy, efficiency, and explainability. Unlike traditional diffusion-based models, DMSM eliminates the dependency on training from fully sampled data, making it more practical for real-world clinical settings. We evaluated DMSM on two human MRI datasets, demonstrating that it achieves favorable performance over several supervised and self-supervised baselines, particularly in preserving fine anatomical structures and suppressing artifacts under high acceleration factors. Additionally, our model generates uncertainty maps that correlate reasonably well with reconstruction errors, offering valuable clinically interpretable guidance and potentially enhancing diagnostic confidence.
FairREAD: Re-fusing Demographic Attributes after Disentanglement for Fair Medical Image Classification
Dec 20, 2024Abstract:Recent advancements in deep learning have shown transformative potential in medical imaging, yet concerns about fairness persist due to performance disparities across demographic subgroups. Existing methods aim to address these biases by mitigating sensitive attributes in image data; however, these attributes often carry clinically relevant information, and their removal can compromise model performance-a highly undesirable outcome. To address this challenge, we propose Fair Re-fusion After Disentanglement (FairREAD), a novel, simple, and efficient framework that mitigates unfairness by re-integrating sensitive demographic attributes into fair image representations. FairREAD employs orthogonality constraints and adversarial training to disentangle demographic information while using a controlled re-fusion mechanism to preserve clinically relevant details. Additionally, subgroup-specific threshold adjustments ensure equitable performance across demographic groups. Comprehensive evaluations on a large-scale clinical X-ray dataset demonstrate that FairREAD significantly reduces unfairness metrics while maintaining diagnostic accuracy, establishing a new benchmark for fairness and performance in medical image classification.
Beyond the Eye: A Relational Model for Early Dementia Detection Using Retinal OCTA Images
Aug 09, 2024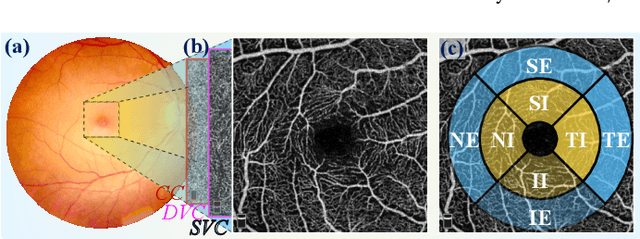
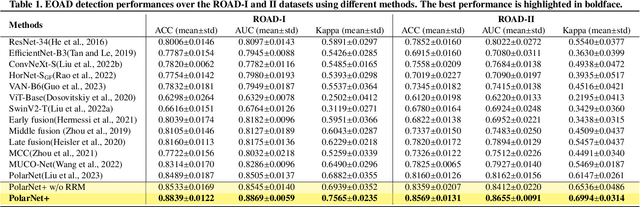
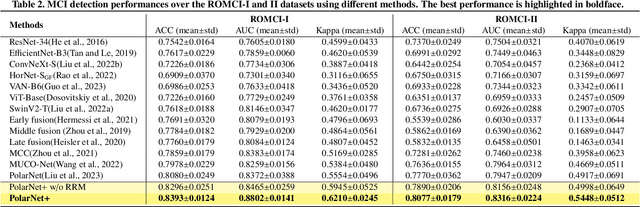
Abstract:Early detection of dementia, such as Alzheimer's disease (AD) or mild cognitive impairment (MCI), is essential to enable timely intervention and potential treatment. Accurate detection of AD/MCI is challenging due to the high complexity, cost, and often invasive nature of current diagnostic techniques, which limit their suitability for large-scale population screening. Given the shared embryological origins and physiological characteristics of the retina and brain, retinal imaging is emerging as a potentially rapid and cost-effective alternative for the identification of individuals with or at high risk of AD. In this paper, we present a novel PolarNet+ that uses retinal optical coherence tomography angiography (OCTA) to discriminate early-onset AD (EOAD) and MCI subjects from controls. Our method first maps OCTA images from Cartesian coordinates to polar coordinates, allowing approximate sub-region calculation to implement the clinician-friendly early treatment of diabetic retinopathy study (ETDRS) grid analysis. We then introduce a multi-view module to serialize and analyze the images along three dimensions for comprehensive, clinically useful information extraction. Finally, we abstract the sequence embedding into a graph, transforming the detection task into a general graph classification problem. A regional relationship module is applied after the multi-view module to excavate the relationship between the sub-regions. Such regional relationship analyses validate known eye-brain links and reveal new discriminative patterns.
Polar-Net: A Clinical-Friendly Model for Alzheimer's Disease Detection in OCTA Images
Nov 10, 2023Abstract:Optical Coherence Tomography Angiography (OCTA) is a promising tool for detecting Alzheimer's disease (AD) by imaging the retinal microvasculature. Ophthalmologists commonly use region-based analysis, such as the ETDRS grid, to study OCTA image biomarkers and understand the correlation with AD. However, existing studies have used general deep computer vision methods, which present challenges in providing interpretable results and leveraging clinical prior knowledge. To address these challenges, we propose a novel deep-learning framework called Polar-Net. Our approach involves mapping OCTA images from Cartesian coordinates to polar coordinates, which allows for the use of approximate sector convolution and enables the implementation of the ETDRS grid-based regional analysis method commonly used in clinical practice. Furthermore, Polar-Net incorporates clinical prior information of each sector region into the training process, which further enhances its performance. Additionally, our framework adapts to acquire the importance of the corresponding retinal region, which helps researchers and clinicians understand the model's decision-making process in detecting AD and assess its conformity to clinical observations. Through evaluations on private and public datasets, we have demonstrated that Polar-Net outperforms existing state-of-the-art methods and provides more valuable pathological evidence for the association between retinal vascular changes and AD. In addition, we also show that the two innovative modules introduced in our framework have a significant impact on improving overall performance.
Retinal Structure Detection in OCTA Image via Voting-based Multi-task Learning
Aug 23, 2022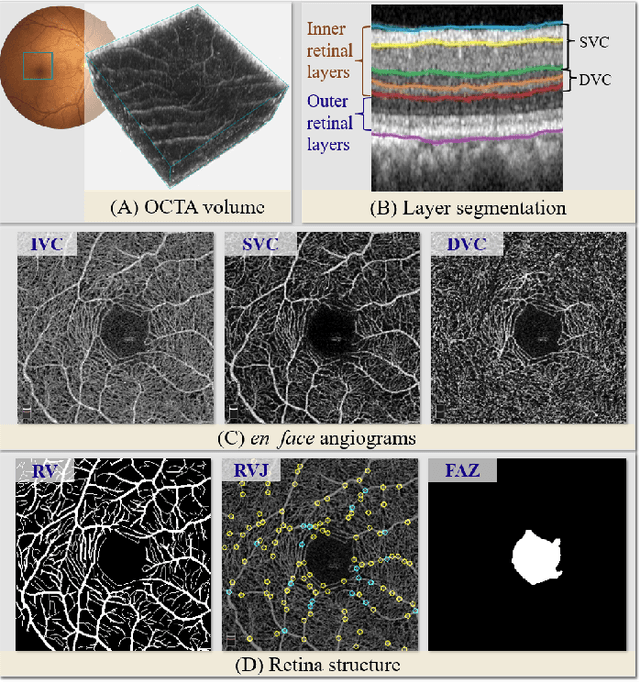
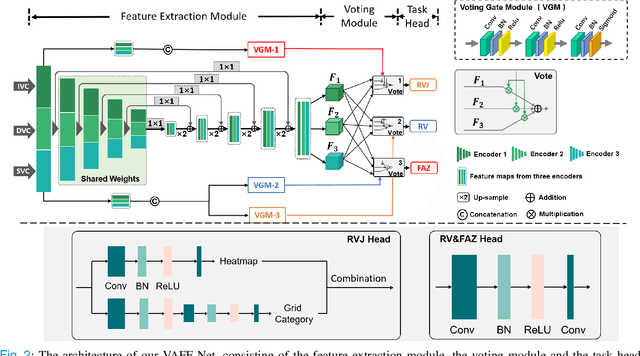
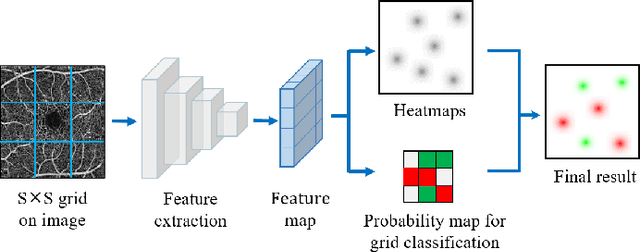
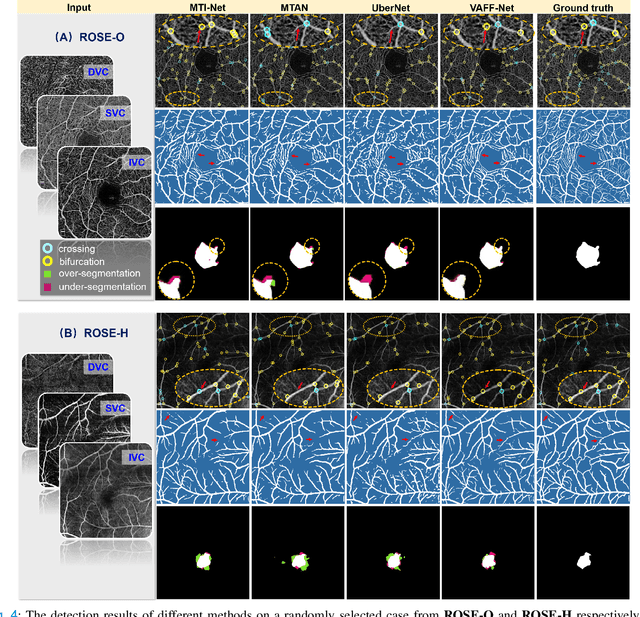
Abstract:Automated detection of retinal structures, such as retinal vessels (RV), the foveal avascular zone (FAZ), and retinal vascular junctions (RVJ), are of great importance for understanding diseases of the eye and clinical decision-making. In this paper, we propose a novel Voting-based Adaptive Feature Fusion multi-task network (VAFF-Net) for joint segmentation, detection, and classification of RV, FAZ, and RVJ in optical coherence tomography angiography (OCTA). A task-specific voting gate module is proposed to adaptively extract and fuse different features for specific tasks at two levels: features at different spatial positions from a single encoder, and features from multiple encoders. In particular, since the complexity of the microvasculature in OCTA images makes simultaneous precise localization and classification of retinal vascular junctions into bifurcation/crossing a challenging task, we specifically design a task head by combining the heatmap regression and grid classification. We take advantage of three different \textit{en face} angiograms from various retinal layers, rather than following existing methods that use only a single \textit{en face}. To facilitate further research, part of these datasets with the source code and evaluation benchmark have been released for public access:https://github.com/iMED-Lab/VAFF-Net.
3D Vessel Reconstruction in OCT-Angiography via Depth Map Estimation
Feb 26, 2021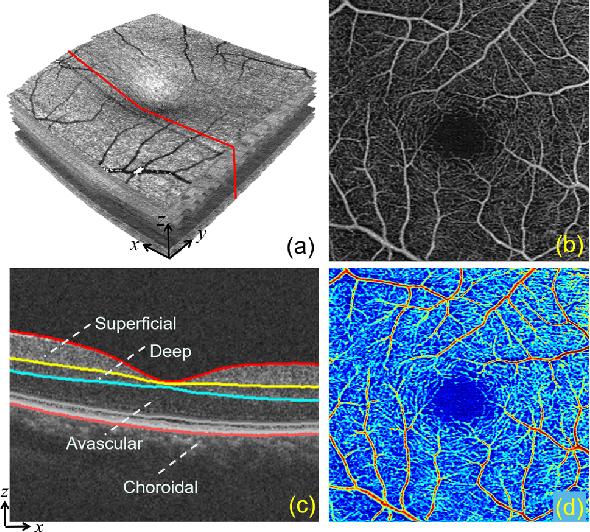
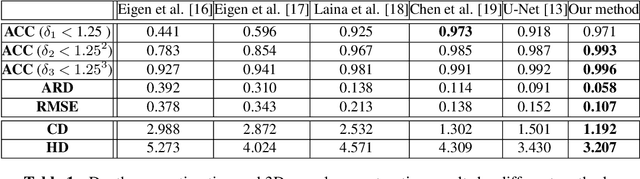
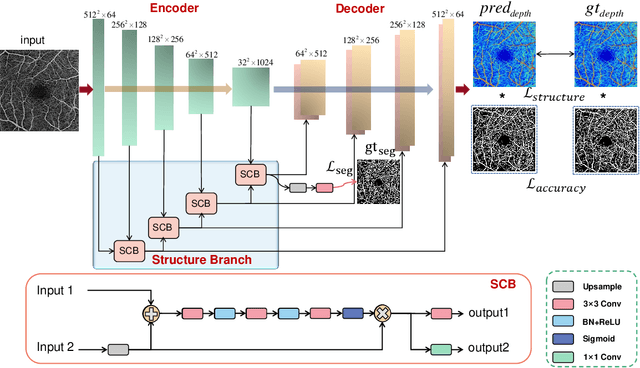
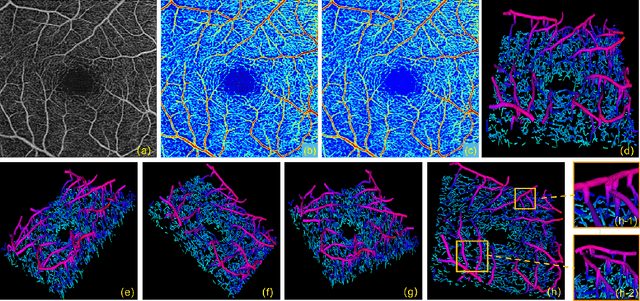
Abstract:Optical Coherence Tomography Angiography (OCTA) has been increasingly used in the management of eye and systemic diseases in recent years. Manual or automatic analysis of blood vessel in 2D OCTA images (en face angiograms) is commonly used in clinical practice, however it may lose rich 3D spatial distribution information of blood vessels or capillaries that are useful for clinical decision-making. In this paper, we introduce a novel 3D vessel reconstruction framework based on the estimation of vessel depth maps from OCTA images. First, we design a network with structural constraints to predict the depth of blood vessels in OCTA images. In order to promote the accuracy of the predicted depth map at both the overall structure- and pixel- level, we combine MSE and SSIM loss as the training loss function. Finally, the 3D vessel reconstruction is achieved by utilizing the estimated depth map and 2D vessel segmentation results. Experimental results demonstrate that our method is effective in the depth prediction and 3D vessel reconstruction for OCTA images.% results may be used to guide subsequent vascular analysis
Reconstruction and Quantification of 3D Iris Surface for Angle-Closure Glaucoma Detection in Anterior Segment OCT
Jun 09, 2020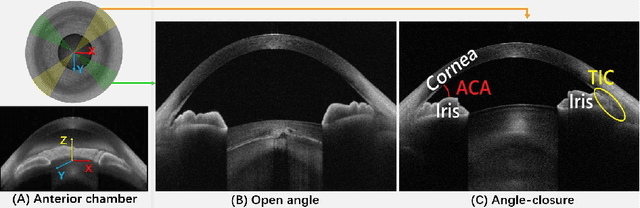
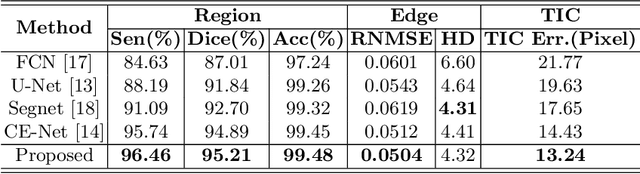
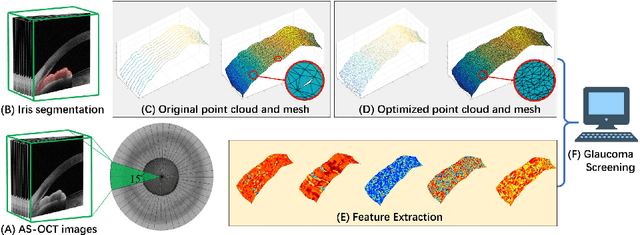
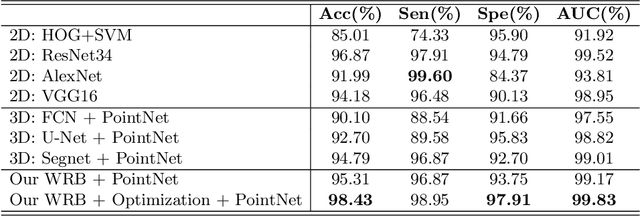
Abstract:Precise characterization and analysis of iris shape from Anterior Segment OCT (AS-OCT) are of great importance in facilitating diagnosis of angle-closure-related diseases. Existing methods focus solely on analyzing structural properties identified from the 2D slice, while accurate characterization of morphological changes of iris shape in 3D AS-OCT may be able to reveal in addition the risk of disease progression. In this paper, we propose a novel framework for reconstruction and quantification of 3D iris surface from AS-OCT imagery. We consider it to be the first work to detect angle-closure glaucoma by means of 3D representation. An iris segmentation network with wavelet refinement block (WRB) is first proposed to generate the initial shape of the iris from single AS-OCT slice. The 3D iris surface is then reconstructed using a guided optimization method with Poisson-disk sampling. Finally, a set of surface-based features are extracted, which are used in detecting of angle-closure glaucoma. Experimental results demonstrate that our method is highly effective in iris segmentation and surface reconstruction. Moreover, we show that 3D-based representation achieves better performance in angle-closure glaucoma detection than does 2D-based feature.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge