Jialun Wu
iEmoTTS: Toward Robust Cross-Speaker Emotion Transfer and Control for Speech Synthesis based on Disentanglement between Prosody and Timbre
Jun 29, 2022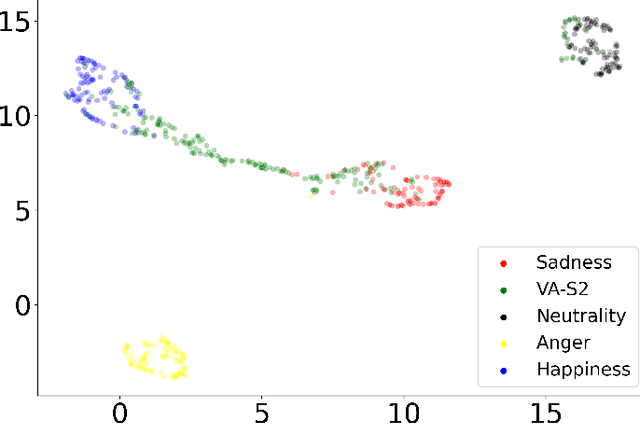
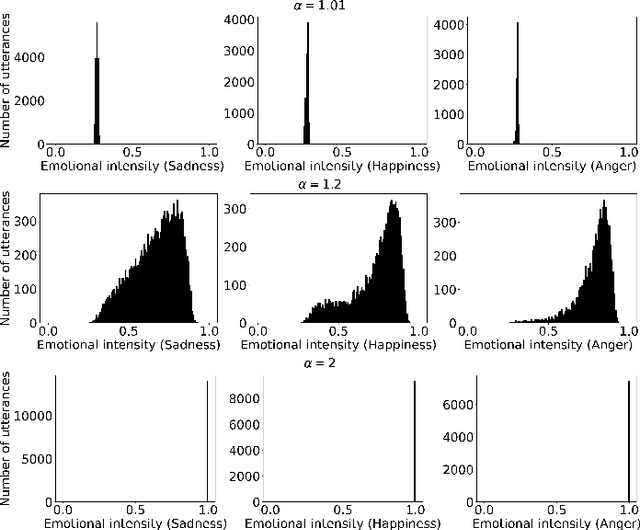
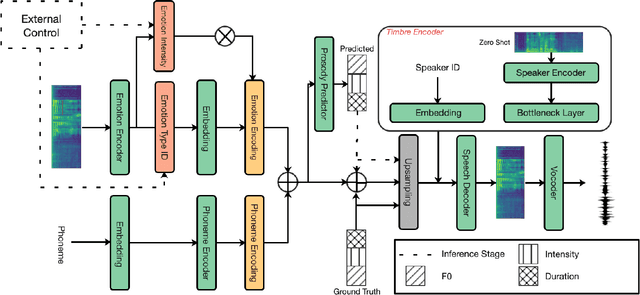
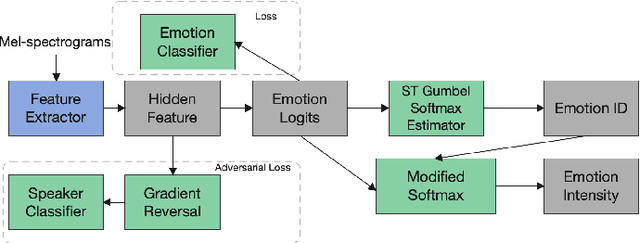
Abstract:The capability of generating speech with specific type of emotion is desired for many applications of human-computer interaction. Cross-speaker emotion transfer is a common approach to generating emotional speech when speech with emotion labels from target speakers is not available for model training. This paper presents a novel cross-speaker emotion transfer system, named iEmoTTS. The system is composed of an emotion encoder, a prosody predictor, and a timbre encoder. The emotion encoder extracts the identity of emotion type as well as the respective emotion intensity from the mel-spectrogram of input speech. The emotion intensity is measured by the posterior probability that the input utterance carries that emotion. The prosody predictor is used to provide prosodic features for emotion transfer. The timber encoder provides timbre-related information for the system. Unlike many other studies which focus on disentangling speaker and style factors of speech, the iEmoTTS is designed to achieve cross-speaker emotion transfer via disentanglement between prosody and timbre. Prosody is considered as the main carrier of emotion-related speech characteristics and timbre accounts for the essential characteristics for speaker identification. Zero-shot emotion transfer, meaning that speech of target speakers are not seen in model training, is also realized with iEmoTTS. Extensive experiments of subjective evaluation have been carried out. The results demonstrate the effectiveness of iEmoTTS as compared with other recently proposed systems of cross-speaker emotion transfer. It is shown that iEmoTTS can produce speech with designated emotion type and controllable emotion intensity. With appropriate information bottleneck capacity, iEmoTTS is able to effectively transfer emotion information to a new speaker. Audio samples are publicly available\footnote{https://patrick-g-zhang.github.io/iemotts/}.
PIMIP: An Open Source Platform for Pathology Information Management and Integration
Nov 09, 2021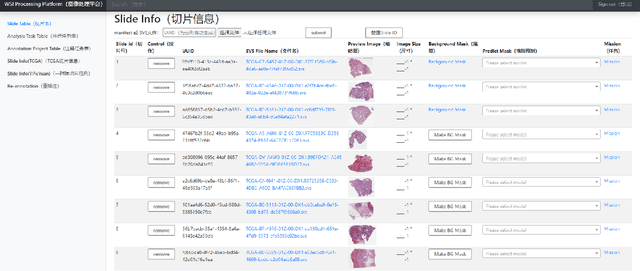
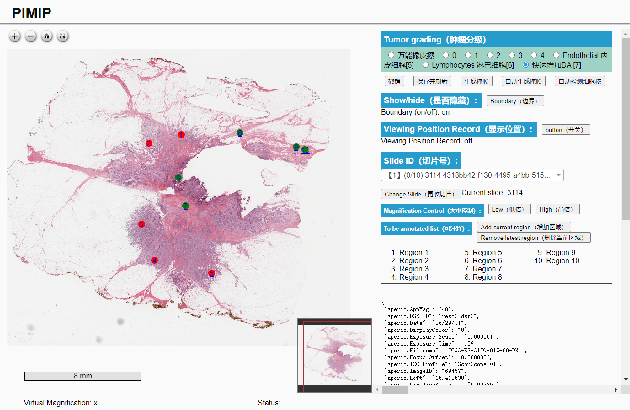
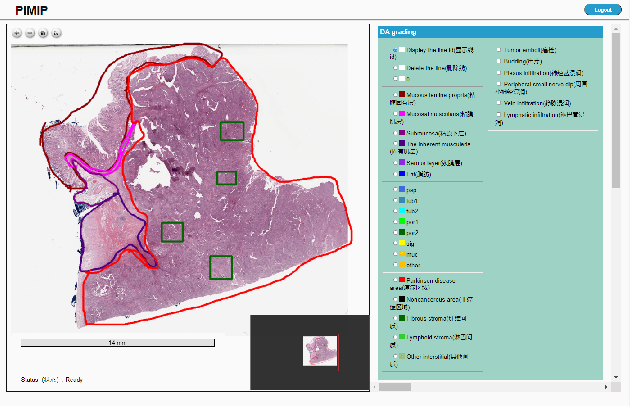
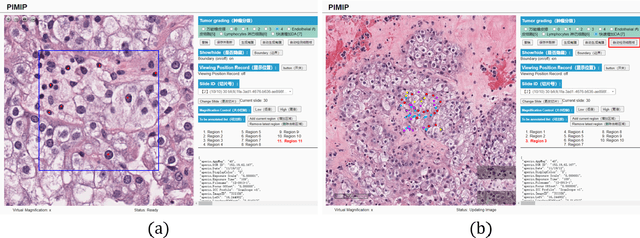
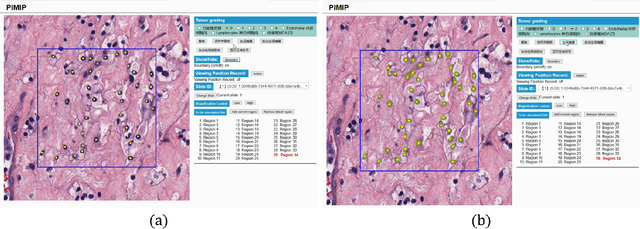
Abstract:Digital pathology plays a crucial role in the development of artificial intelligence in the medical field. The digital pathology platform can make the pathological resources digital and networked, and realize the permanent storage of visual data and the synchronous browsing processing without the limitation of time and space. It has been widely used in various fields of pathology. However, there is still a lack of an open and universal digital pathology platform to assist doctors in the management and analysis of digital pathological sections, as well as the management and structured description of relevant patient information. Most platforms cannot integrate image viewing, annotation and analysis, and text information management. To solve the above problems, we propose a comprehensive and extensible platform PIMIP. Our PIMIP has developed the image annotation functions based on the visualization of digital pathological sections. Our annotation functions support multi-user collaborative annotation and multi-device annotation, and realize the automation of some annotation tasks. In the annotation task, we invited a professional pathologist for guidance. We introduce a machine learning module for image analysis. The data we collected included public data from local hospitals and clinical examples. Our platform is more clinical and suitable for clinical use. In addition to image data, we also structured the management and display of text information. So our platform is comprehensive. The platform framework is built in a modular way to support users to add machine learning modules independently, which makes our platform extensible.
BioIE: Biomedical Information Extraction with Multi-head Attention Enhanced Graph Convolutional Network
Oct 26, 2021
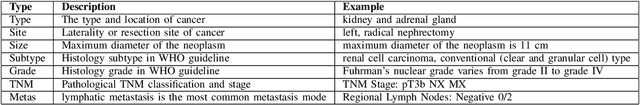
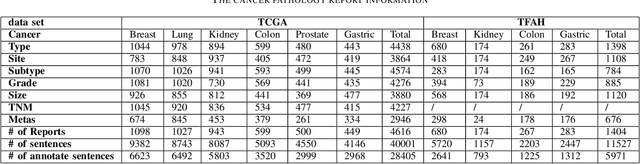
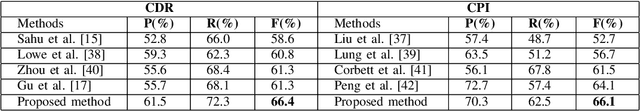
Abstract:Constructing large-scaled medical knowledge graphs can significantly boost healthcare applications for medical surveillance, bring much attention from recent research. An essential step in constructing large-scale MKG is extracting information from medical reports. Recently, information extraction techniques have been proposed and show promising performance in biomedical information extraction. However, these methods only consider limited types of entity and relation due to the noisy biomedical text data with complex entity correlations. Thus, they fail to provide enough information for constructing MKGs and restrict the downstream applications. To address this issue, we propose Biomedical Information Extraction, a hybrid neural network to extract relations from biomedical text and unstructured medical reports. Our model utilizes a multi-head attention enhanced graph convolutional network to capture the complex relations and context information while resisting the noise from the data. We evaluate our model on two major biomedical relationship extraction tasks, chemical-disease relation and chemical-protein interaction, and a cross-hospital pan-cancer pathology report corpus. The results show that our method achieves superior performance than baselines. Furthermore, we evaluate the applicability of our method under a transfer learning setting and show that BioIE achieves promising performance in processing medical text from different formats and writing styles.
A Personalized Diagnostic Generation Framework Based on Multi-source Heterogeneous Data
Oct 26, 2021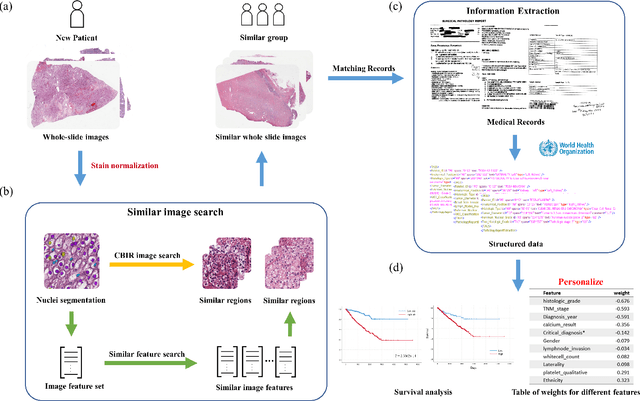
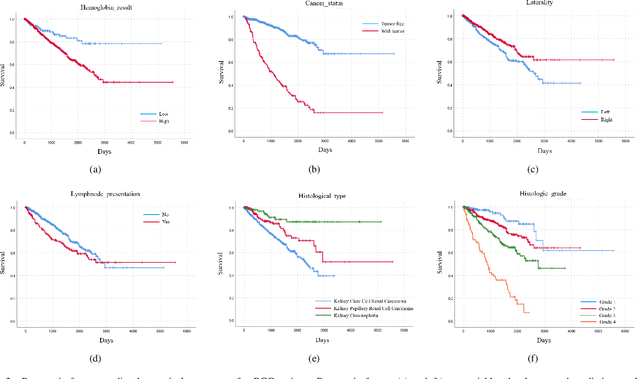
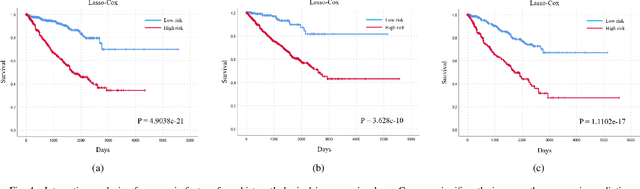
Abstract:Personalized diagnoses have not been possible due to sear amount of data pathologists have to bear during the day-to-day routine. This lead to the current generalized standards that are being continuously updated as new findings are reported. It is noticeable that these effective standards are developed based on a multi-source heterogeneous data, including whole-slide images and pathology and clinical reports. In this study, we propose a framework that combines pathological images and medical reports to generate a personalized diagnosis result for individual patient. We use nuclei-level image feature similarity and content-based deep learning method to search for a personalized group of population with similar pathological characteristics, extract structured prognostic information from descriptive pathology reports of the similar patient population, and assign importance of different prognostic factors to generate a personalized pathological diagnosis result. We use multi-source heterogeneous data from TCGA (The Cancer Genome Atlas) database. The result demonstrate that our framework matches the performance of pathologists in the diagnosis of renal cell carcinoma. This framework is designed to be generic, thus could be applied for other types of cancer. The weights could provide insights to the known prognostic factors and further guide more precise clinical treatment protocols.
W-Net: A Two-Stage Convolutional Network for Nucleus Detection in Histopathology Image
Oct 26, 2021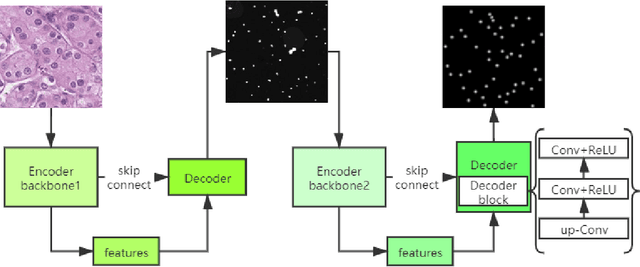
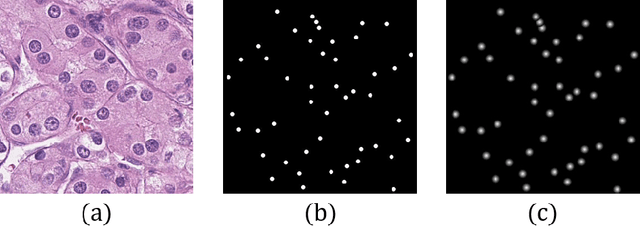
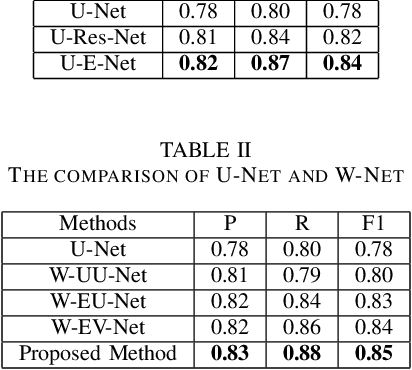
Abstract:Pathological diagnosis is the gold standard for cancer diagnosis, but it is labor-intensive, in which tasks such as cell detection, classification, and counting are particularly prominent. A common solution for automating these tasks is using nucleus segmentation technology. However, it is hard to train a robust nucleus segmentation model, due to several challenging problems, the nucleus adhesion, stacking, and excessive fusion with the background. Recently, some researchers proposed a series of automatic nucleus segmentation methods based on point annotation, which can significant improve the model performance. Nevertheless, the point annotation needs to be marked by experienced pathologists. In order to take advantage of segmentation methods based on point annotation, further alleviate the manual workload, and make cancer diagnosis more efficient and accurate, it is necessary to develop an automatic nucleus detection algorithm, which can automatically and efficiently locate the position of the nucleus in the pathological image and extract valuable information for pathologists. In this paper, we propose a W-shaped network for automatic nucleus detection. Different from the traditional U-Net based method, mapping the original pathology image to the target mask directly, our proposed method split the detection task into two sub-tasks. The first sub-task maps the original pathology image to the binary mask, then the binary mask is mapped to the density mask in the second sub-task. After the task is split, the task's difficulty is significantly reduced, and the network's overall performance is improved.
A Precision Diagnostic Framework of Renal Cell Carcinoma on Whole-Slide Images using Deep Learning
Oct 26, 2021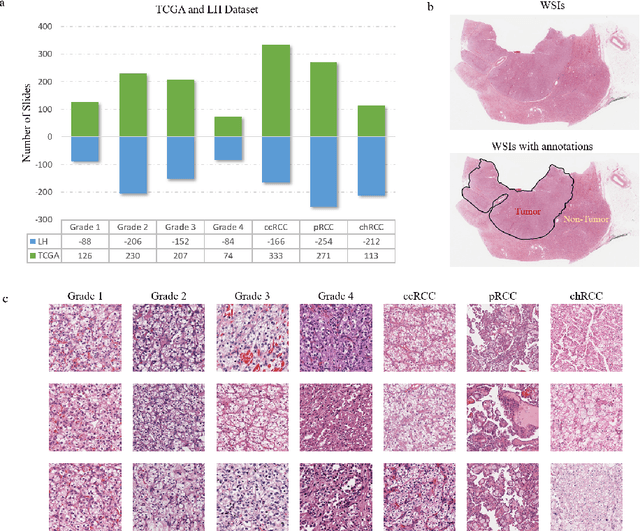
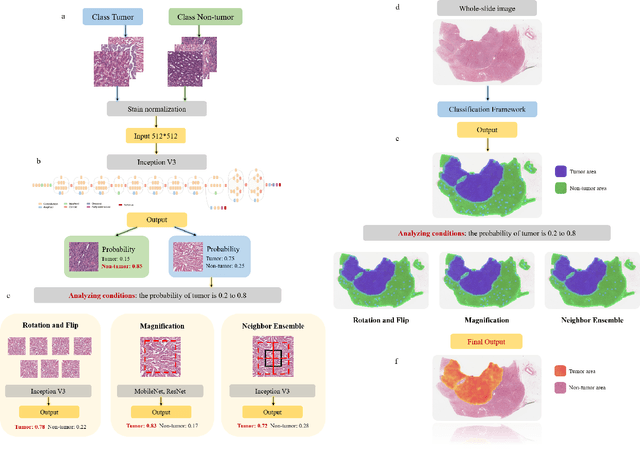
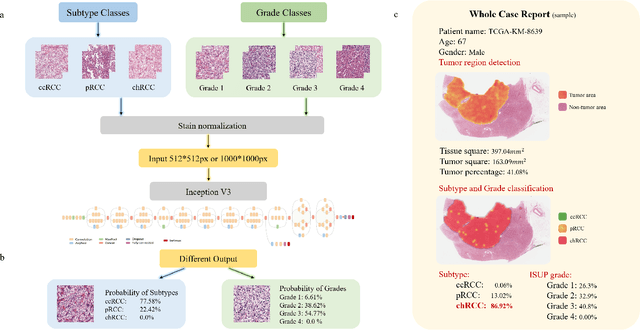
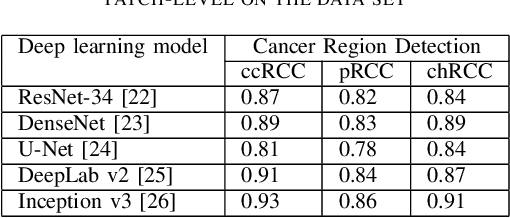
Abstract:Diagnostic pathology, which is the basis and gold standard of cancer diagnosis, provides essential information on the prognosis of the disease and vital evidence for clinical treatment. Tumor region detection, subtype and grade classification are the fundamental diagnostic indicators for renal cell carcinoma (RCC) in whole-slide images (WSIs). However, pathological diagnosis is subjective, differences in observation and diagnosis between pathologists is common in hospitals with inadequate diagnostic capacity. The main challenge for developing deep learning based RCC diagnostic system is the lack of large-scale datasets with precise annotations. In this work, we proposed a deep learning-based framework for analyzing histopathological images of patients with renal cell carcinoma, which has the potential to achieve pathologist-level accuracy in diagnosis. A deep convolutional neural network (InceptionV3) was trained on the high-quality annotated dataset of The Cancer Genome Atlas (TCGA) whole-slide histopathological image for accurate tumor area detection, classification of RCC subtypes, and ISUP grades classification of clear cell carcinoma subtypes. These results suggest that our framework can help pathologists in the detection of cancer region and classification of subtypes and grades, which could be applied to any cancer type, providing auxiliary diagnosis and promoting clinical consensus.
Instance-based Vision Transformer for Subtyping of Papillary Renal Cell Carcinoma in Histopathological Image
Jun 23, 2021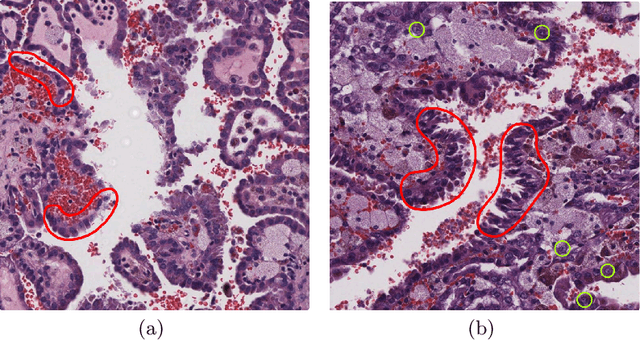
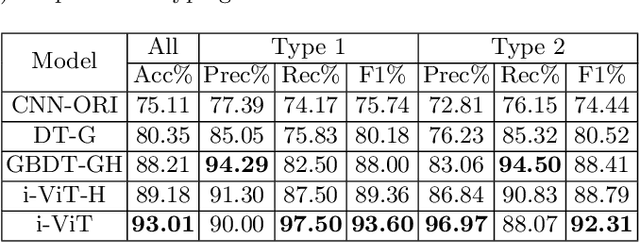
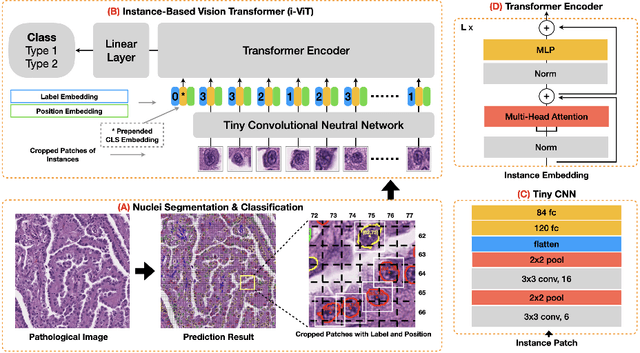
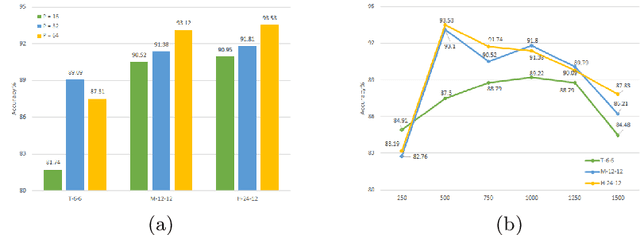
Abstract:Histological subtype of papillary (p) renal cell carcinoma (RCC), type 1 vs. type 2, is an essential prognostic factor. The two subtypes of pRCC have a similar pattern, i.e., the papillary architecture, yet some subtle differences, including cellular and cell-layer level patterns. However, the cellular and cell-layer level patterns almost cannot be captured by existing CNN-based models in large-size histopathological images, which brings obstacles to directly applying these models to such a fine-grained classification task. This paper proposes a novel instance-based Vision Transformer (i-ViT) to learn robust representations of histopathological images for the pRCC subtyping task by extracting finer features from instance patches (by cropping around segmented nuclei and assigning predicted grades). The proposed i-ViT takes top-K instances as input and aggregates them for capturing both the cellular and cell-layer level patterns by a position-embedding layer, a grade-embedding layer, and a multi-head multi-layer self-attention module. To evaluate the performance of the proposed framework, experienced pathologists are invited to selected 1162 regions of interest from 171 whole slide images of type 1 and type 2 pRCC. Experimental results show that the proposed method achieves better performance than existing CNN-based models with a significant margin.
Nuclei Grading of Clear Cell Renal Cell Carcinoma in Histopathological Image by Composite High-Resolution Network
Jun 20, 2021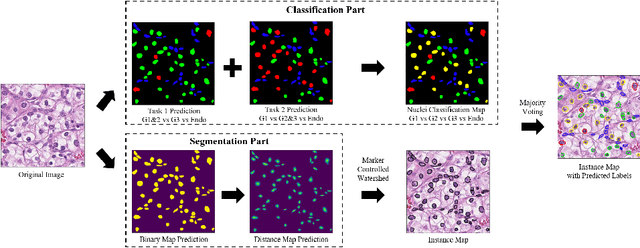
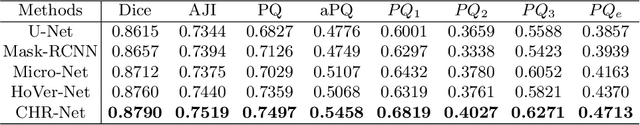
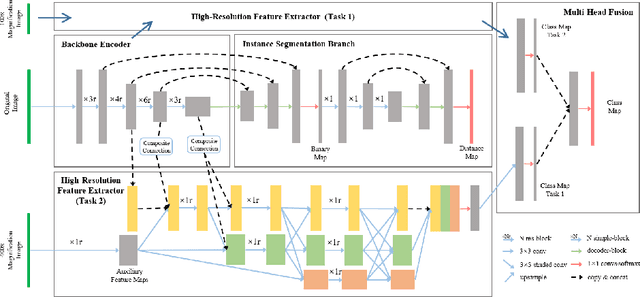
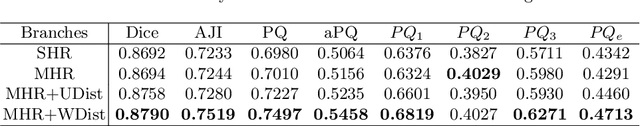
Abstract:The grade of clear cell renal cell carcinoma (ccRCC) is a critical prognostic factor, making ccRCC nuclei grading a crucial task in RCC pathology analysis. Computer-aided nuclei grading aims to improve pathologists' work efficiency while reducing their misdiagnosis rate by automatically identifying the grades of tumor nuclei within histopathological images. Such a task requires precisely segment and accurately classify the nuclei. However, most of the existing nuclei segmentation and classification methods can not handle the inter-class similarity property of nuclei grading, thus can not be directly applied to the ccRCC grading task. In this paper, we propose a Composite High-Resolution Network for ccRCC nuclei grading. Specifically, we propose a segmentation network called W-Net that can separate the clustered nuclei. Then, we recast the fine-grained classification of nuclei to two cross-category classification tasks, based on two high-resolution feature extractors (HRFEs) which are proposed for learning these two tasks. The two HRFEs share the same backbone encoder with W-Net by a composite connection so that meaningful features for the segmentation task can be inherited for the classification task. Last, a head-fusion block is applied to generate the predicted label of each nucleus. Furthermore, we introduce a dataset for ccRCC nuclei grading, containing 1000 image patches with 70945 annotated nuclei. We demonstrate that our proposed method achieves state-of-the-art performance compared to existing methods on this large ccRCC grading dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge