Izabela Horvath
SELMA3D challenge: Self-supervised learning for 3D light-sheet microscopy image segmentation
Jan 07, 2025Abstract:Recent innovations in light sheet microscopy, paired with developments in tissue clearing techniques, enable the 3D imaging of large mammalian tissues with cellular resolution. Combined with the progress in large-scale data analysis, driven by deep learning, these innovations empower researchers to rapidly investigate the morphological and functional properties of diverse biological samples. Segmentation, a crucial preliminary step in the analysis process, can be automated using domain-specific deep learning models with expert-level performance. However, these models exhibit high sensitivity to domain shifts, leading to a significant drop in accuracy when applied to data outside their training distribution. To address this limitation, and inspired by the recent success of self-supervised learning in training generalizable models, we organized the SELMA3D Challenge during the MICCAI 2024 conference. SELMA3D provides a vast collection of light-sheet images from cleared mice and human brains, comprising 35 large 3D images-each with over 1000^3 voxels-and 315 annotated small patches for finetuning, preliminary testing and final testing. The dataset encompasses diverse biological structures, including vessel-like and spot-like structures. Five teams participated in all phases of the challenge, and their proposed methods are reviewed in this paper. Quantitative and qualitative results from most participating teams demonstrate that self-supervised learning on large datasets improves segmentation model performance and generalization. We will continue to support and extend SELMA3D as an inaugural MICCAI challenge focused on self-supervised learning for 3D microscopy image segmentation.
The Brain Tumor Segmentation Challenge 2023: Local Synthesis of Healthy Brain Tissue via Inpainting
May 15, 2023



Abstract:A myriad of algorithms for the automatic analysis of brain MR images is available to support clinicians in their decision-making. For brain tumor patients, the image acquisition time series typically starts with a scan that is already pathological. This poses problems, as many algorithms are designed to analyze healthy brains and provide no guarantees for images featuring lesions. Examples include but are not limited to algorithms for brain anatomy parcellation, tissue segmentation, and brain extraction. To solve this dilemma, we introduce the BraTS 2023 inpainting challenge. Here, the participants' task is to explore inpainting techniques to synthesize healthy brain scans from lesioned ones. The following manuscript contains the task formulation, dataset, and submission procedure. Later it will be updated to summarize the findings of the challenge. The challenge is organized as part of the BraTS 2023 challenge hosted at the MICCAI 2023 conference in Vancouver, Canada.
Deep Quality Estimation: Creating Surrogate Models for Human Quality Ratings
May 17, 2022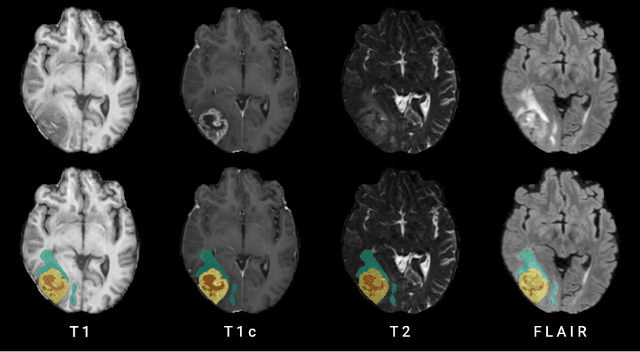
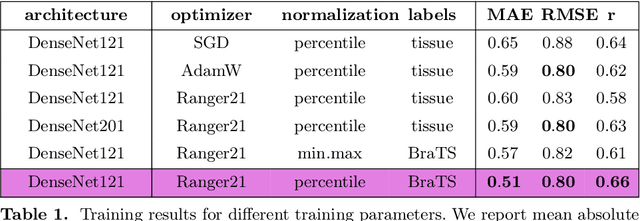
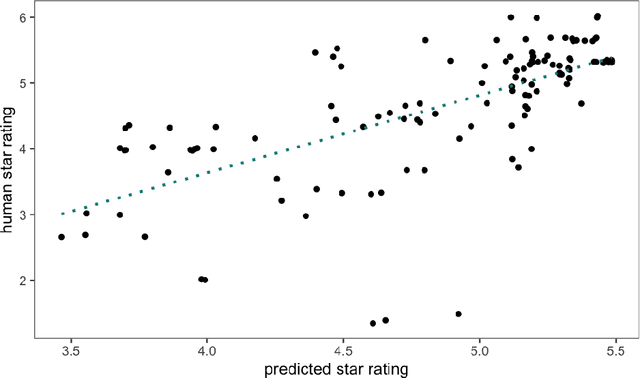
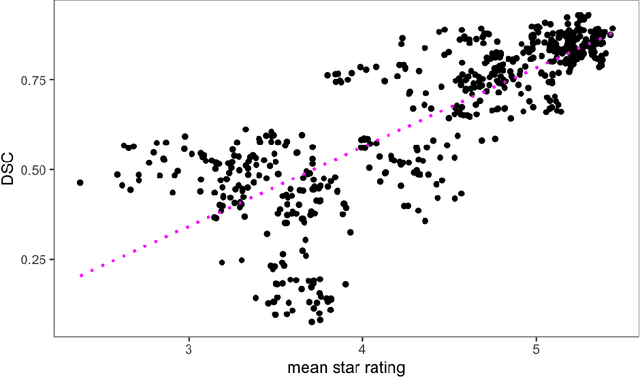
Abstract:Human ratings are abstract representations of segmentation quality. To approximate human quality ratings on scarce expert data, we train surrogate quality estimation models. We evaluate on a complex multi-class segmentation problem, specifically glioma segmentation following the BraTS annotation protocol. The training data features quality ratings from 15 expert neuroradiologists on a scale ranging from 1 to 6 stars for various computer-generated and manual 3D annotations. Even though the networks operate on 2D images and with scarce training data, we can approximate segmentation quality within a margin of error comparable to human intra-rater reliability. Segmentation quality prediction has broad applications. While an understanding of segmentation quality is imperative for successful clinical translation of automatic segmentation quality algorithms, it can play an essential role in training new segmentation models. Due to the split-second inference times, it can be directly applied within a loss function or as a fully-automatic dataset curation mechanism in a federated learning setting.
blob loss: instance imbalance aware loss functions for semantic segmentation
May 17, 2022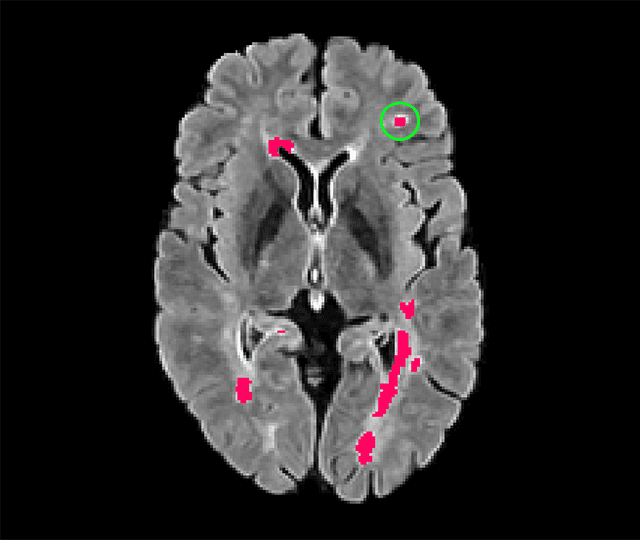
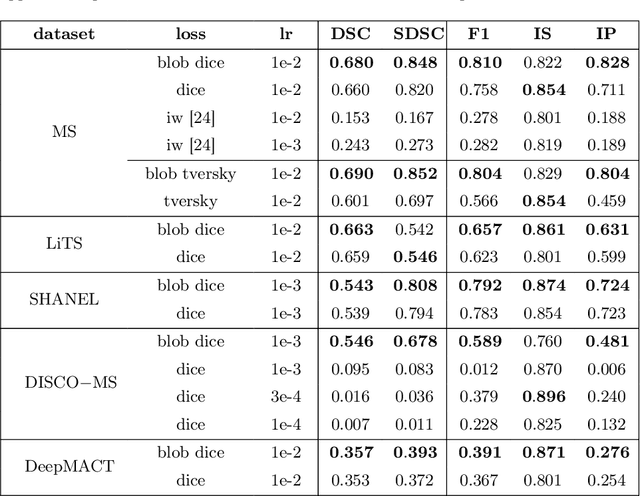

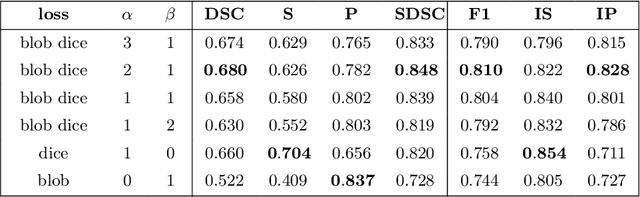
Abstract:Deep convolutional neural networks have proven to be remarkably effective in semantic segmentation tasks. Most popular loss functions were introduced targeting improved volumetric scores, such as the Sorensen Dice coefficient. By design, DSC can tackle class imbalance; however, it does not recognize instance imbalance within a class. As a result, a large foreground instance can dominate minor instances and still produce a satisfactory Sorensen Dice coefficient. Nevertheless, missing out on instances will lead to poor detection performance. This represents a critical issue in applications such as disease progression monitoring. For example, it is imperative to locate and surveil small-scale lesions in the follow-up of multiple sclerosis patients. We propose a novel family of loss functions, nicknamed blob loss, primarily aimed at maximizing instance-level detection metrics, such as F1 score and sensitivity. Blob loss is designed for semantic segmentation problems in which the instances are the connected components within a class. We extensively evaluate a DSC-based blob loss in five complex 3D semantic segmentation tasks featuring pronounced instance heterogeneity in terms of texture and morphology. Compared to soft Dice loss, we achieve 5 percent improvement for MS lesions, 3 percent improvement for liver tumor, and an average 2 percent improvement for Microscopy segmentation tasks considering F1 score.
METGAN: Generative Tumour Inpainting and Modality Synthesis in Light Sheet Microscopy
Apr 23, 2021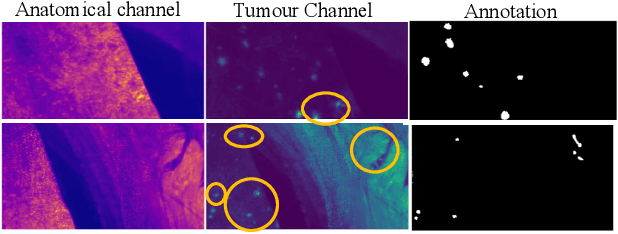
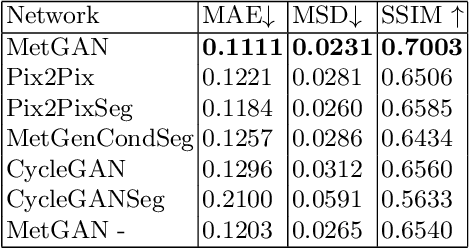
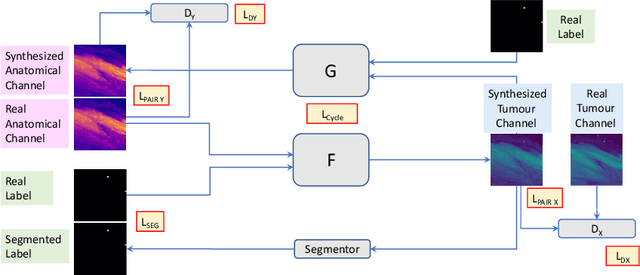
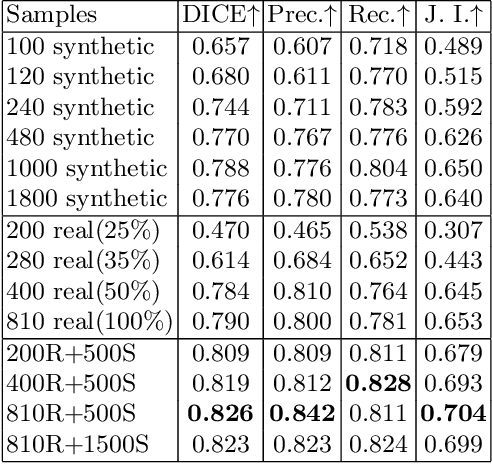
Abstract:Novel multimodal imaging methods are capable of generating extensive, super high resolution datasets for preclinical research. Yet, a massive lack of annotations prevents the broad use of deep learning to analyze such data. So far, existing generative models fail to mitigate this problem because of frequent labeling errors. In this paper, we introduce a novel generative method which leverages real anatomical information to generate realistic image-label pairs of tumours. We construct a dual-pathway generator, for the anatomical image and label, trained in a cycle-consistent setup, constrained by an independent, pretrained segmentor. The generated images yield significant quantitative improvement compared to existing methods. To validate the quality of synthesis, we train segmentation networks on a dataset augmented with the synthetic data, substantially improving the segmentation over baseline.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge