Oliver Schoppe
METGAN: Generative Tumour Inpainting and Modality Synthesis in Light Sheet Microscopy
Apr 23, 2021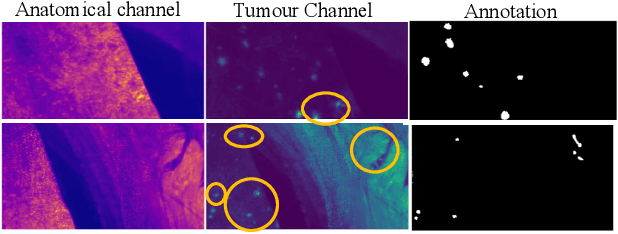
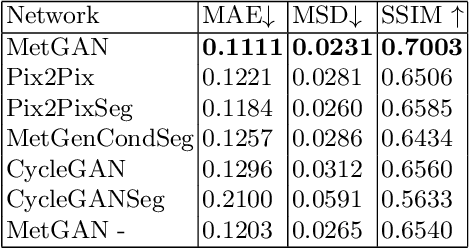
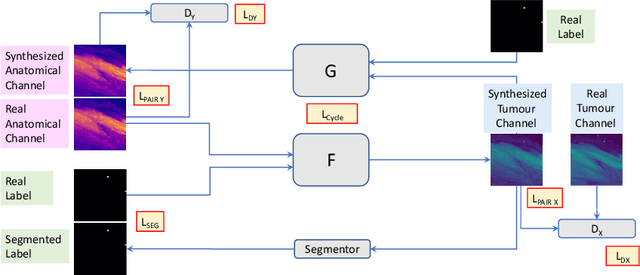
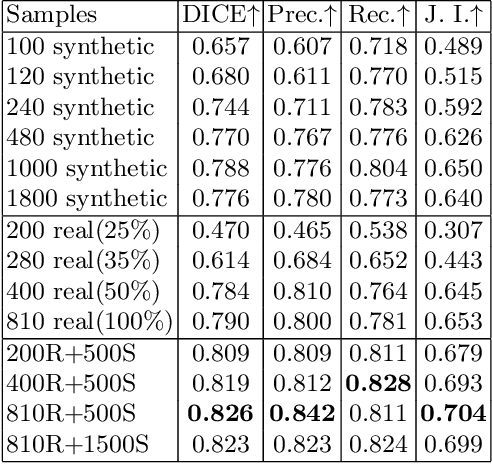
Abstract:Novel multimodal imaging methods are capable of generating extensive, super high resolution datasets for preclinical research. Yet, a massive lack of annotations prevents the broad use of deep learning to analyze such data. So far, existing generative models fail to mitigate this problem because of frequent labeling errors. In this paper, we introduce a novel generative method which leverages real anatomical information to generate realistic image-label pairs of tumours. We construct a dual-pathway generator, for the anatomical image and label, trained in a cycle-consistent setup, constrained by an independent, pretrained segmentor. The generated images yield significant quantitative improvement compared to existing methods. To validate the quality of synthesis, we train segmentation networks on a dataset augmented with the synthetic data, substantially improving the segmentation over baseline.
Red-GAN: Attacking class imbalance via conditioned generation. Yet another medical imaging perspective
Apr 30, 2020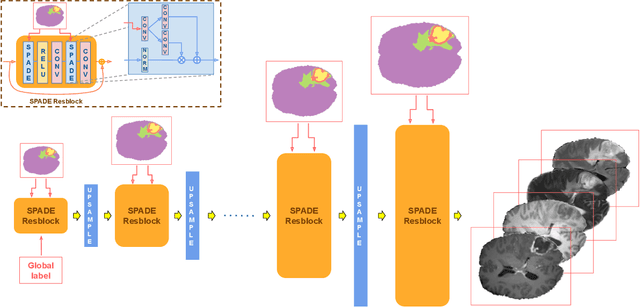

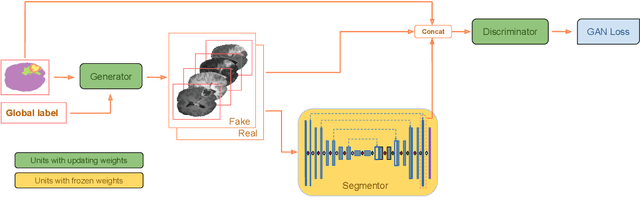
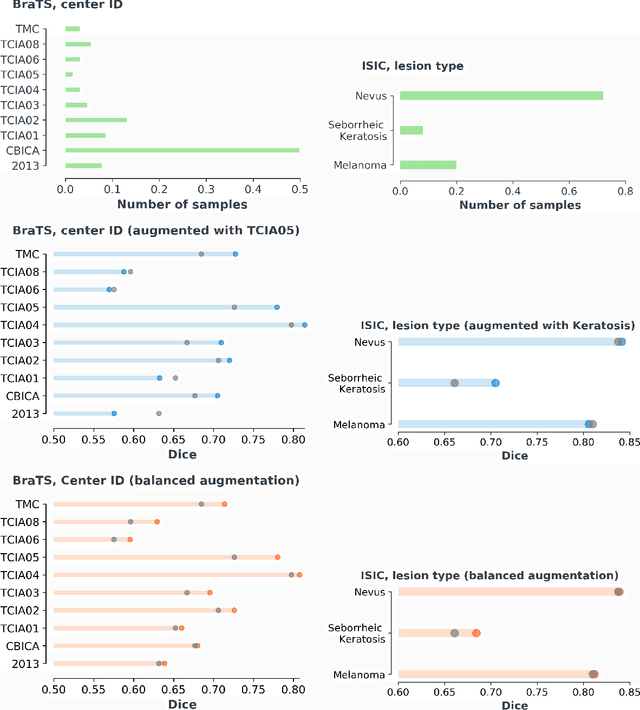
Abstract:Exploiting learning algorithms under scarce data regimes is a limitation and a reality of the medical imaging field. In an attempt to mitigate the problem, we propose a data augmentation protocol based on generative adversarial networks. We condition the networks at a pixel-level (segmentation mask) and at a global-level information (acquisition environment or lesion type). Such conditioning provides immediate access to the image-label pairs while controlling global class specific appearance of the synthesized images. To stimulate synthesis of the features relevant for the segmentation task, an additional passive player in a form of segmentor is introduced into the the adversarial game. We validate the approach on two medical datasets: BraTS, ISIC. By controlling the class distribution through injection of synthetic images into the training set we achieve control over the accuracy levels of the datasets' classes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge