Timo Loehr
Self-pruning Graph Neural Network for Predicting Inflammatory Disease Activity in Multiple Sclerosis from Brain MR Images
Aug 31, 2023



Abstract:Multiple Sclerosis (MS) is a severe neurological disease characterized by inflammatory lesions in the central nervous system. Hence, predicting inflammatory disease activity is crucial for disease assessment and treatment. However, MS lesions can occur throughout the brain and vary in shape, size and total count among patients. The high variance in lesion load and locations makes it challenging for machine learning methods to learn a globally effective representation of whole-brain MRI scans to assess and predict disease. Technically it is non-trivial to incorporate essential biomarkers such as lesion load or spatial proximity. Our work represents the first attempt to utilize graph neural networks (GNN) to aggregate these biomarkers for a novel global representation. We propose a two-stage MS inflammatory disease activity prediction approach. First, a 3D segmentation network detects lesions, and a self-supervised algorithm extracts their image features. Second, the detected lesions are used to build a patient graph. The lesions act as nodes in the graph and are initialized with image features extracted in the first stage. Finally, the lesions are connected based on their spatial proximity and the inflammatory disease activity prediction is formulated as a graph classification task. Furthermore, we propose a self-pruning strategy to auto-select the most critical lesions for prediction. Our proposed method outperforms the existing baseline by a large margin (AUCs of 0.67 vs. 0.61 and 0.66 vs. 0.60 for one-year and two-year inflammatory disease activity, respectively). Finally, our proposed method enjoys inherent explainability by assigning an importance score to each lesion for the overall prediction. Code is available at https://github.com/chinmay5/ms_ida.git
blob loss: instance imbalance aware loss functions for semantic segmentation
May 17, 2022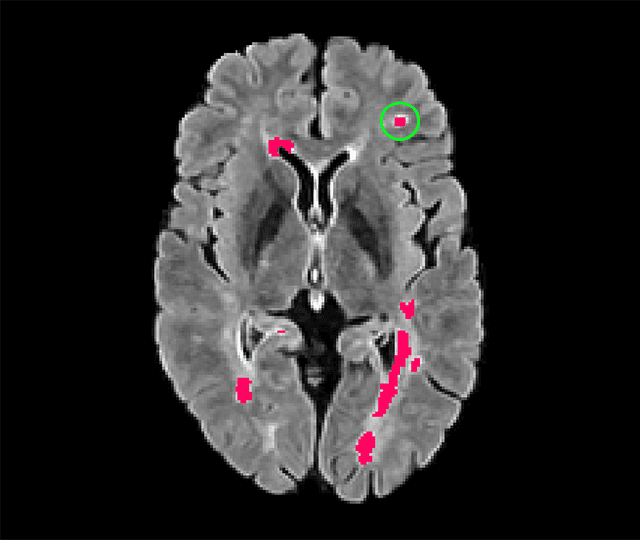
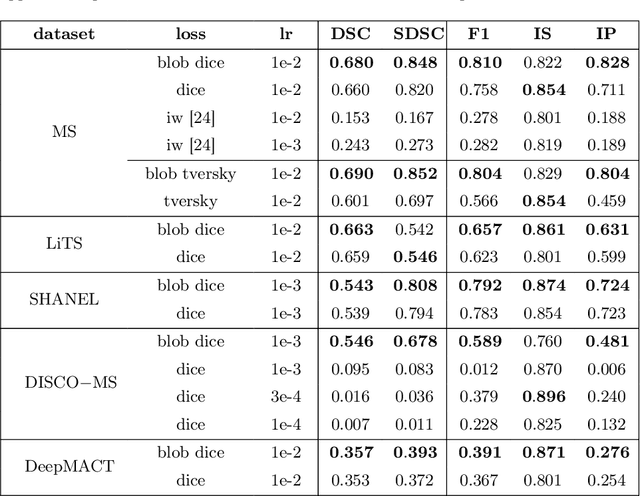

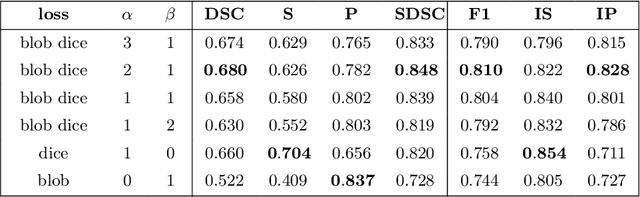
Abstract:Deep convolutional neural networks have proven to be remarkably effective in semantic segmentation tasks. Most popular loss functions were introduced targeting improved volumetric scores, such as the Sorensen Dice coefficient. By design, DSC can tackle class imbalance; however, it does not recognize instance imbalance within a class. As a result, a large foreground instance can dominate minor instances and still produce a satisfactory Sorensen Dice coefficient. Nevertheless, missing out on instances will lead to poor detection performance. This represents a critical issue in applications such as disease progression monitoring. For example, it is imperative to locate and surveil small-scale lesions in the follow-up of multiple sclerosis patients. We propose a novel family of loss functions, nicknamed blob loss, primarily aimed at maximizing instance-level detection metrics, such as F1 score and sensitivity. Blob loss is designed for semantic segmentation problems in which the instances are the connected components within a class. We extensively evaluate a DSC-based blob loss in five complex 3D semantic segmentation tasks featuring pronounced instance heterogeneity in terms of texture and morphology. Compared to soft Dice loss, we achieve 5 percent improvement for MS lesions, 3 percent improvement for liver tumor, and an average 2 percent improvement for Microscopy segmentation tasks considering F1 score.
FedCostWAvg: A new averaging for better Federated Learning
Nov 16, 2021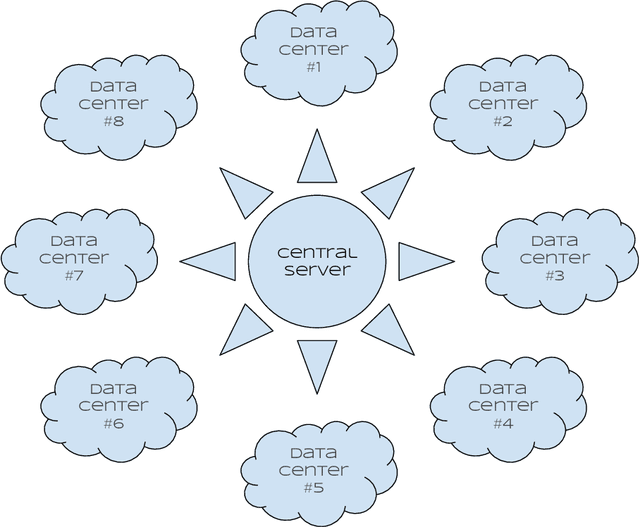

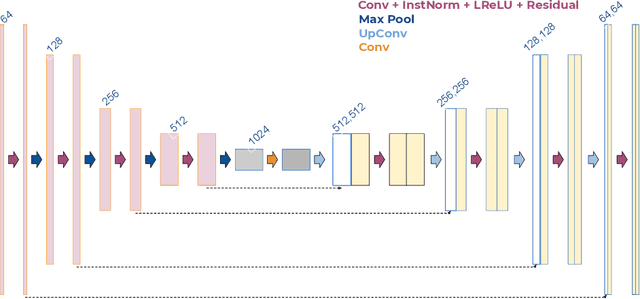

Abstract:We propose a simple new aggregation strategy for federated learning that won the MICCAI Federated Tumor Segmentation Challenge 2021 (FETS), the first ever challenge on Federated Learning in the Machine Learning community. Our method addresses the problem of how to aggregate multiple models that were trained on different data sets. Conceptually, we propose a new way to choose the weights when averaging the different models, thereby extending the current state of the art (FedAvg). Empirical validation demonstrates that our approach reaches a notable improvement in segmentation performance compared to FedAvg.
e-UDA: Efficient Unsupervised Domain Adaptation for Cross-Site Medical Image Segmentation
Jan 25, 2020



Abstract:Domain adaptation in healthcare data is a potentially critical component in making computer-aided diagnostic systems applicable cross multiple sites and imaging scanners. In this paper, we propose an efficient unsupervised domain adaptation framework for robust image segmentation cross multiple similar domains. We enforce our algorithm to not only adapt to the new domains via an adversarial optimization, rejecting unlikely segmentation patterns, but also to maintain its performance on the source training data, by incorporating both semantic and boundary information into the data distributions. Further, as we do not have labels for the transfer domain, we propose a new quality score for the adaptation process, and strategies to retrain the diagnostic algorithm in a stable fashion. Using multi-centric data from a public benchmark for brain lesion segmentation, we demonstrate that recalibrating on just few unlabeled image sets from the target domain improves segmentation accuracies drastically, with performances almost similar to those from algorithms trained on fresh and fully annotated data from the test domain.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge