Hongying Feng
Diffusion Transformer-based Universal Dose Denoising for Pencil Beam Scanning Proton Therapy
Jun 04, 2025Abstract:Purpose: Intensity-modulated proton therapy (IMPT) offers precise tumor coverage while sparing organs at risk (OARs) in head and neck (H&N) cancer. However, its sensitivity to anatomical changes requires frequent adaptation through online adaptive radiation therapy (oART), which depends on fast, accurate dose calculation via Monte Carlo (MC) simulations. Reducing particle count accelerates MC but degrades accuracy. To address this, denoising low-statistics MC dose maps is proposed to enable fast, high-quality dose generation. Methods: We developed a diffusion transformer-based denoising framework. IMPT plans and 3D CT images from 80 H&N patients were used to generate noisy and high-statistics dose maps using MCsquare (1 min and 10 min per plan, respectively). Data were standardized into uniform chunks with zero-padding, normalized, and transformed into quasi-Gaussian distributions. Testing was done on 10 H&N, 10 lung, 10 breast, and 10 prostate cancer cases, preprocessed identically. The model was trained with noisy dose maps and CT images as input and high-statistics dose maps as ground truth, using a combined loss of mean square error (MSE), residual loss, and regional MAE (focusing on top/bottom 10% dose voxels). Performance was assessed via MAE, 3D Gamma passing rate, and DVH indices. Results: The model achieved MAEs of 0.195 (H&N), 0.120 (lung), 0.172 (breast), and 0.376 Gy[RBE] (prostate). 3D Gamma passing rates exceeded 92% (3%/2mm) across all sites. DVH indices for clinical target volumes (CTVs) and OARs closely matched the ground truth. Conclusion: A diffusion transformer-based denoising framework was developed and, though trained only on H&N data, generalizes well across multiple disease sites.
The Radiation Oncology NLP Database
Jan 19, 2024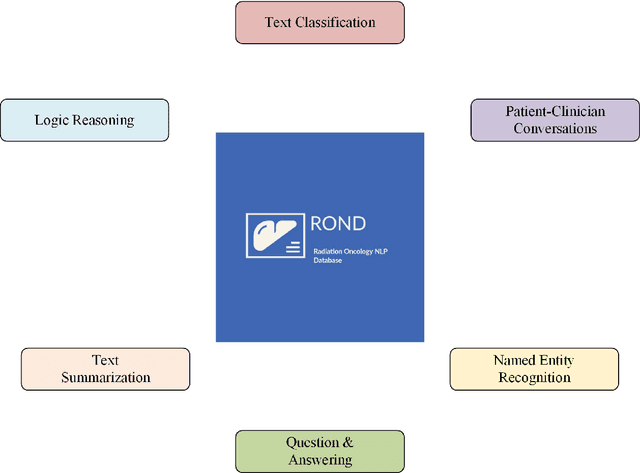
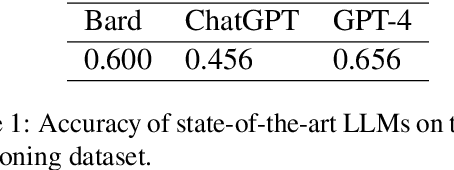
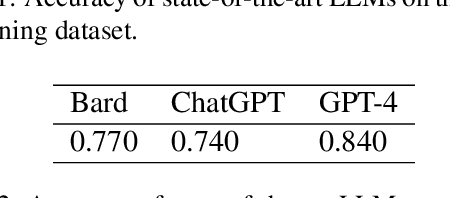
Abstract:We present the Radiation Oncology NLP Database (ROND), the first dedicated Natural Language Processing (NLP) dataset for radiation oncology, an important medical specialty that has received limited attention from the NLP community in the past. With the advent of Artificial General Intelligence (AGI), there is an increasing need for specialized datasets and benchmarks to facilitate research and development. ROND is specifically designed to address this gap in the domain of radiation oncology, a field that offers many opportunities for NLP exploration. It encompasses various NLP tasks including Logic Reasoning, Text Classification, Named Entity Recognition (NER), Question Answering (QA), Text Summarization, and Patient-Clinician Conversations, each with a distinct focus on radiation oncology concepts and application cases. In addition, we have developed an instruction-tuning dataset consisting of over 20k instruction pairs (based on ROND) and trained a large language model, CancerChat. This serves to demonstrate the potential of instruction-tuning large language models within a highly-specialized medical domain. The evaluation results in this study could serve as baseline results for future research. ROND aims to stimulate advancements in radiation oncology and clinical NLP by offering a platform for testing and improving algorithms and models in a domain-specific context. The ROND dataset is a joint effort of multiple U.S. health institutions. The data is available at https://github.com/zl-liu/Radiation-Oncology-NLP-Database.
Noisy probing dose facilitated dose prediction for pencil beam scanning proton therapy: physics enhances generalizability
Dec 02, 2023



Abstract:Purpose: Prior AI-based dose prediction studies in photon and proton therapy often neglect underlying physics, limiting their generalizability to handle outlier clinical cases, especially for pencil beam scanning proton therapy (PBSPT). Our aim is to design a physics-aware and generalizable AI-based PBSPT dose prediction method that has the underlying physics considered to achieve high generalizability to properly handle the outlier clinical cases. Methods and Materials: This study analyzed PBSPT plans of 103 prostate and 78 lung cancer patients from our institution,with each case comprising CT images, structure sets, and plan doses from our Monte-Carlo dose engine (serving as the ground truth). Three methods were evaluated in the ablation study: the ROI-based method, the beam mask and sliding window method, and the noisy probing dose method. Twelve cases with uncommon beam angles or prescription doses tested the methods' generalizability to rare treatment planning scenarios. Performance evaluation used DVH indices, 3D Gamma passing rates (3%/2mm/10%), and dice coefficients for dose agreement. Results: The noisy probing dose method showed improved agreement of DVH indices, 3D Gamma passing rates, and dice coefficients compared to the conventional methods for the testing cases. The noisy probing dose method showed better generalizability in the 6 outlier cases than the ROI-based and beam mask-based methods with 3D Gamma passing rates (for prostate cancer, targets: 89.32%$\pm$1.45% vs. 93.48%$\pm$1.51% vs. 96.79%$\pm$0.83%, OARs: 85.87%$\pm$1.73% vs. 91.15%$\pm$1.13% vs. 94.29%$\pm$1.01%). The dose predictions were completed within 0.3 seconds. Conclusions: We've devised a novel noisy probing dose method for PBSPT dose prediction in prostate and lung cancer patients. With more physics included, it enhances the generalizability of dose prediction in handling outlier clinical cases.
Benchmarking a foundation LLM on its ability to re-label structure names in accordance with the AAPM TG-263 report
Oct 05, 2023



Abstract:Purpose: To introduce the concept of using large language models (LLMs) to re-label structure names in accordance with the American Association of Physicists in Medicine (AAPM) Task Group (TG)-263 standard, and to establish a benchmark for future studies to reference. Methods and Materials: The Generative Pre-trained Transformer (GPT)-4 application programming interface (API) was implemented as a Digital Imaging and Communications in Medicine (DICOM) storage server, which upon receiving a structure set DICOM file, prompts GPT-4 to re-label the structure names of both target volumes and normal tissues according to the AAPM TG-263. Three disease sites, prostate, head and neck, and thorax were selected for evaluation. For each disease site category, 150 patients were randomly selected for manually tuning the instructions prompt (in batches of 50) and 50 patients were randomly selected for evaluation. Structure names that were considered were those that were most likely to be relevant for studies utilizing structure contours for many patients. Results: The overall re-labeling accuracy of both target volumes and normal tissues for prostate, head and neck, and thorax cases was 96.0%, 98.5%, and 96.9% respectively. Re-labeling of target volumes was less accurate on average except for prostate - 100%, 93.1%, and 91.1% respectively. Conclusions: Given the accuracy of GPT-4 in re-labeling structure names of both target volumes and normal tissues as presented in this work, LLMs are poised to be the preferred method for standardizing structure names in radiation oncology, especially considering the rapid advancements in LLM capabilities that are likely to continue.
Segment Anything Model (SAM) for Radiation Oncology
Jul 04, 2023
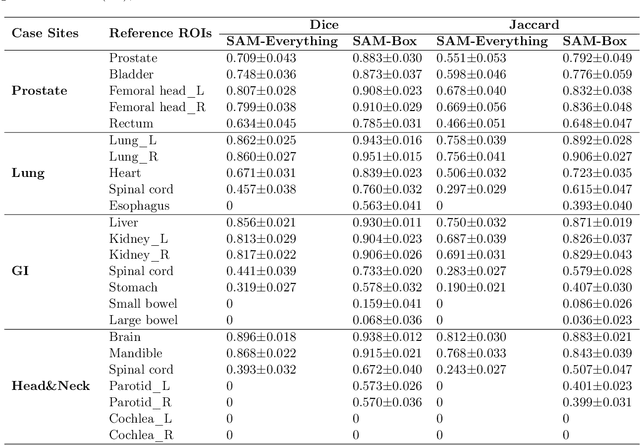


Abstract:In this study, we evaluate the performance of the Segment Anything Model (SAM) in clinical radiotherapy. Our results indicate that SAM's 'segment anything' mode can achieve clinically acceptable segmentation results in most organs-at-risk (OARs) with Dice scores higher than 0.7. SAM's 'box prompt' mode further improves the Dice scores by 0.1 to 0.5. Considering the size of the organ and the clarity of its boundary, SAM displays better performance for large organs with clear boundaries but performs worse for smaller organs with unclear boundaries. Given that SAM, a model pre-trained purely on natural images, can handle the delineation of OARs from medical images with clinically acceptable accuracy, these results highlight SAM's robust generalization capabilities with consistent accuracy in automatic segmentation for radiotherapy. In other words, SAM can achieve delineation of different OARs at different sites using a generic automatic segmentation model. SAM's generalization capabilities across different disease sites suggest that it is technically feasible to develop a generic model for automatic segmentation in radiotherapy.
Deep-Learning-based Fast and Accurate 3D CT Deformable Image Registration in Lung Cancer
Apr 21, 2023Abstract:Purpose: In some proton therapy facilities, patient alignment relies on two 2D orthogonal kV images, taken at fixed, oblique angles, as no 3D on-the-bed imaging is available. The visibility of the tumor in kV images is limited since the patient's 3D anatomy is projected onto a 2D plane, especially when the tumor is behind high-density structures such as bones. This can lead to large patient setup errors. A solution is to reconstruct the 3D CT image from the kV images obtained at the treatment isocenter in the treatment position. Methods: An asymmetric autoencoder-like network built with vision-transformer blocks was developed. The data was collected from 1 head and neck patient: 2 orthogonal kV images (1024x1024 voxels), 1 3D CT with padding (512x512x512) acquired from the in-room CT-on-rails before kVs were taken and 2 digitally-reconstructed-radiograph (DRR) images (512x512) based on the CT. We resampled kV images every 8 voxels and DRR and CT every 4 voxels, thus formed a dataset consisting of 262,144 samples, in which the images have a dimension of 128 for each direction. In training, both kV and DRR images were utilized, and the encoder was encouraged to learn the jointed feature map from both kV and DRR images. In testing, only independent kV images were used. The full-size synthetic CT (sCT) was achieved by concatenating the sCTs generated by the model according to their spatial information. The image quality of the synthetic CT (sCT) was evaluated using mean absolute error (MAE) and per-voxel-absolute-CT-number-difference volume histogram (CDVH). Results: The model achieved a speed of 2.1s and a MAE of <40HU. The CDVH showed that <5% of the voxels had a per-voxel-absolute-CT-number-difference larger than 185 HU. Conclusion: A patient-specific vision-transformer-based network was developed and shown to be accurate and efficient to reconstruct 3D CT images from kV images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge