Gwenole Quellec
Multimodal Information Fusion For The Diagnosis Of Diabetic Retinopathy
Mar 20, 2023


Abstract:Diabetes is a chronic disease characterized by excess sugar in the blood and affects 422 million people worldwide, including 3.3 million in France. One of the frequent complications of diabetes is diabetic retinopathy (DR): it is the leading cause of blindness in the working population of developed countries. As a result, ophthalmology is on the verge of a revolution in screening, diagnosing, and managing of pathologies. This upheaval is led by the arrival of technologies based on artificial intelligence. The "Evaluation intelligente de la r\'etinopathie diab\'etique" (EviRed) project uses artificial intelligence to answer a medical need: replacing the current classification of diabetic retinopathy which is mainly based on outdated fundus photography and providing an insufficient prediction precision. EviRed exploits modern fundus imaging devices and artificial intelligence to properly integrate the vast amount of data they provide with other available medical data of the patient. The goal is to improve diagnosis and prediction and help ophthalmologists to make better decisions during diabetic retinopathy follow-up. In this study, we investigate the fusion of different modalities acquired simultaneously with a PLEXElite 9000 (Carl Zeiss Meditec Inc. Dublin, California, USA), namely 3-D structural optical coherence tomography (OCT), 3-D OCT angiography (OCTA) and 2-D Line Scanning Ophthalmoscope (LSO), for the automatic detection of proliferative DR.
ADAM Challenge: Detecting Age-related Macular Degeneration from Fundus Images
Feb 18, 2022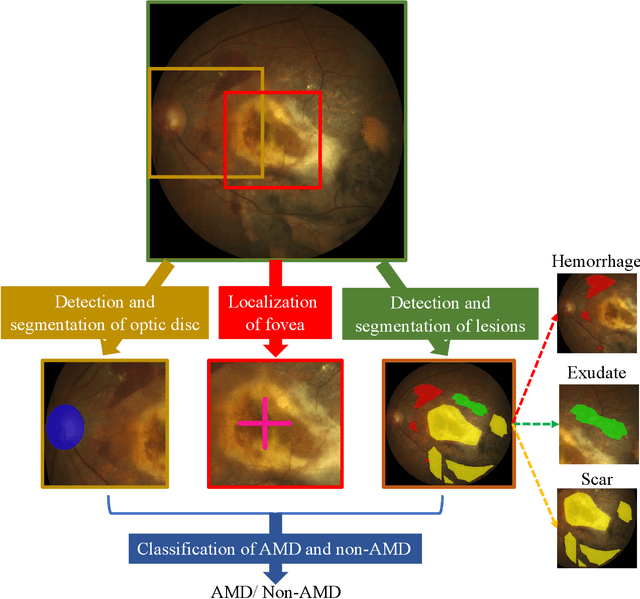
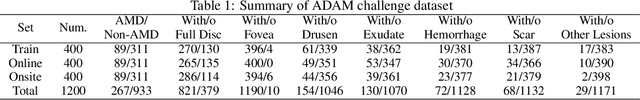
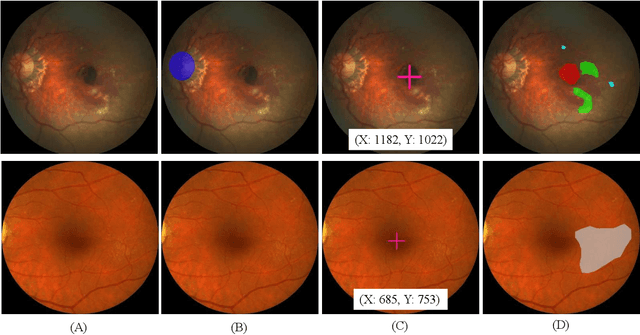
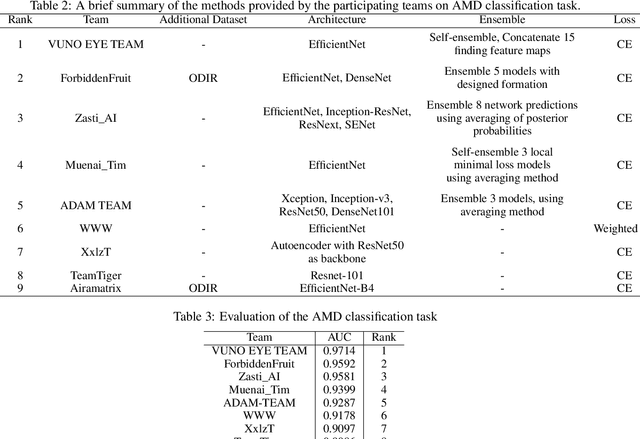
Abstract:Age-related macular degeneration (AMD) is the leading cause of visual impairment among elderly in the world. Early detection of AMD is of great importance as the vision loss caused by AMD is irreversible and permanent. Color fundus photography is the most cost-effective imaging modality to screen for retinal disorders. \textcolor{red}{Recently, some algorithms based on deep learning had been developed for fundus image analysis and automatic AMD detection. However, a comprehensive annotated dataset and a standard evaluation benchmark are still missing.} To deal with this issue, we set up the Automatic Detection challenge on Age-related Macular degeneration (ADAM) for the first time, held as a satellite event of the ISBI 2020 conference. The ADAM challenge consisted of four tasks which cover the main topics in detecting AMD from fundus images, including classification of AMD, detection and segmentation of optic disc, localization of fovea, and detection and segmentation of lesions. The ADAM challenge has released a comprehensive dataset of 1200 fundus images with the category labels of AMD, the pixel-wise segmentation masks of the full optic disc and lesions (drusen, exudate, hemorrhage, scar, and other), as well as the location coordinates of the macular fovea. A uniform evaluation framework has been built to make a fair comparison of different models. During the ADAM challenge, 610 results were submitted for online evaluation, and finally, 11 teams participated in the onsite challenge. This paper introduces the challenge, dataset, and evaluation methods, as well as summarizes the methods and analyzes the results of the participating teams of each task. In particular, we observed that ensembling strategy and clinical prior knowledge can better improve the performances of the deep learning models.
CaDIS: Cataract Dataset for Image Segmentation
Jul 19, 2019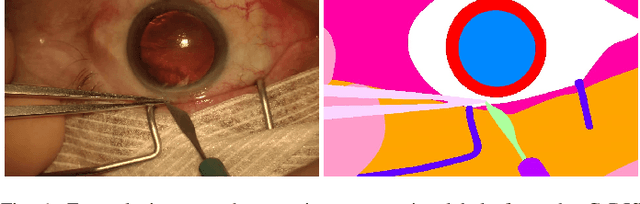
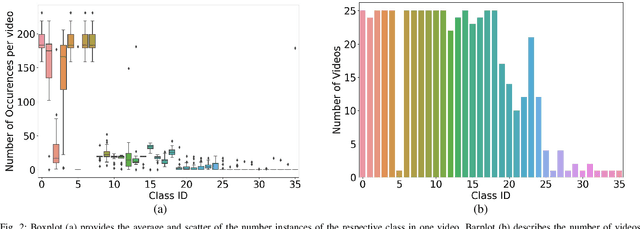
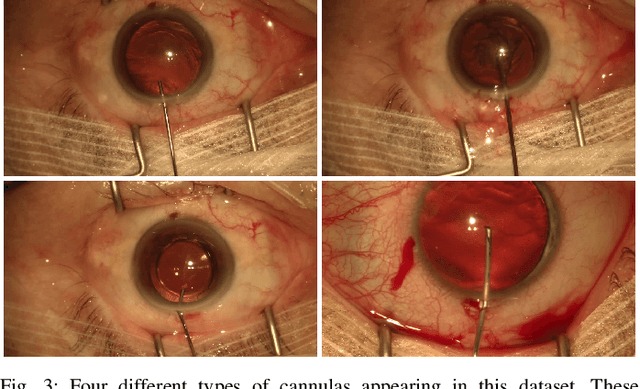
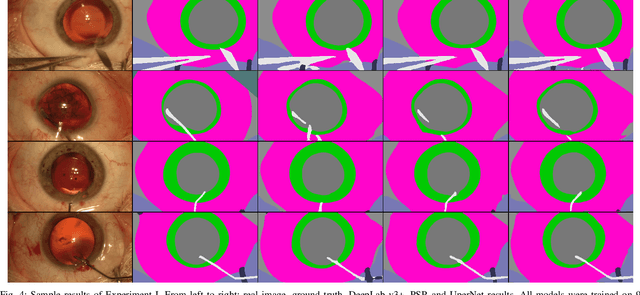
Abstract:Video signals provide a wealth of information about surgical procedures and are the main sensory cue for surgeons. Video processing and understanding can be used to empower computer assisted interventions (CAI) as well as the development of detailed post-operative analysis of the surgical intervention. A fundamental building block to such capabilities is the ability to understand and segment video into semantic labels that differentiate and localize tissue types and different instruments. Deep learning has advanced semantic segmentation techniques dramatically in recent years but is fundamentally reliant on the availability of labelled datasets used to train models. In this paper, we introduce a high quality dataset for semantic segmentation in Cataract surgery. We generated this dataset from the CATARACTS challenge dataset, which is publicly available. To the best of our knowledge, this dataset has the highest quality annotation in surgical data to date. We introduce the dataset and then show the automatic segmentation performance of state-of-the-art models on that dataset as a benchmark.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge