Dale R Webster
LLM-based Text Simplification and its Effect on User Comprehension and Cognitive Load
May 04, 2025Abstract:Information on the web, such as scientific publications and Wikipedia, often surpasses users' reading level. To help address this, we used a self-refinement approach to develop a LLM capability for minimally lossy text simplification. To validate our approach, we conducted a randomized study involving 4563 participants and 31 texts spanning 6 broad subject areas: PubMed (biomedical scientific articles), biology, law, finance, literature/philosophy, and aerospace/computer science. Participants were randomized to viewing original or simplified texts in a subject area, and answered multiple-choice questions (MCQs) that tested their comprehension of the text. The participants were also asked to provide qualitative feedback such as task difficulty. Our results indicate that participants who read the simplified text answered more MCQs correctly than their counterparts who read the original text (3.9% absolute increase, p<0.05). This gain was most striking with PubMed (14.6%), while more moderate gains were observed for finance (5.5%), aerospace/computer science (3.8%) domains, and legal (3.5%). Notably, the results were robust to whether participants could refer back to the text while answering MCQs. The absolute accuracy decreased by up to ~9% for both original and simplified setups where participants could not refer back to the text, but the ~4% overall improvement persisted. Finally, participants' self-reported perceived ease based on a simplified NASA Task Load Index was greater for those who read the simplified text (absolute change on a 5-point scale 0.33, p<0.05). This randomized study, involving an order of magnitude more participants than prior works, demonstrates the potential of LLMs to make complex information easier to understand. Our work aims to enable a broader audience to better learn and make use of expert knowledge available on the web, improving information accessibility.
MINT: A wrapper to make multi-modal and multi-image AI models interactive
Jan 22, 2024Abstract:During the diagnostic process, doctors incorporate multimodal information including imaging and the medical history - and similarly medical AI development has increasingly become multimodal. In this paper we tackle a more subtle challenge: doctors take a targeted medical history to obtain only the most pertinent pieces of information; how do we enable AI to do the same? We develop a wrapper method named MINT (Make your model INTeractive) that automatically determines what pieces of information are most valuable at each step, and ask for only the most useful information. We demonstrate the efficacy of MINT wrapping a skin disease prediction model, where multiple images and a set of optional answers to $25$ standard metadata questions (i.e., structured medical history) are used by a multi-modal deep network to provide a differential diagnosis. We show that MINT can identify whether metadata inputs are needed and if so, which question to ask next. We also demonstrate that when collecting multiple images, MINT can identify if an additional image would be beneficial, and if so, which type of image to capture. We showed that MINT reduces the number of metadata and image inputs needed by 82% and 36.2% respectively, while maintaining predictive performance. Using real-world AI dermatology system data, we show that needing fewer inputs can retain users that may otherwise fail to complete the system submission and drop off without a diagnosis. Qualitative examples show MINT can closely mimic the step-by-step decision making process of a clinical workflow and how this is different for straight forward cases versus more difficult, ambiguous cases. Finally we demonstrate how MINT is robust to different underlying multi-model classifiers and can be easily adapted to user requirements without significant model re-training.
Towards Accurate Differential Diagnosis with Large Language Models
Nov 30, 2023



Abstract:An accurate differential diagnosis (DDx) is a cornerstone of medical care, often reached through an iterative process of interpretation that combines clinical history, physical examination, investigations and procedures. Interactive interfaces powered by Large Language Models (LLMs) present new opportunities to both assist and automate aspects of this process. In this study, we introduce an LLM optimized for diagnostic reasoning, and evaluate its ability to generate a DDx alone or as an aid to clinicians. 20 clinicians evaluated 302 challenging, real-world medical cases sourced from the New England Journal of Medicine (NEJM) case reports. Each case report was read by two clinicians, who were randomized to one of two assistive conditions: either assistance from search engines and standard medical resources, or LLM assistance in addition to these tools. All clinicians provided a baseline, unassisted DDx prior to using the respective assistive tools. Our LLM for DDx exhibited standalone performance that exceeded that of unassisted clinicians (top-10 accuracy 59.1% vs 33.6%, [p = 0.04]). Comparing the two assisted study arms, the DDx quality score was higher for clinicians assisted by our LLM (top-10 accuracy 51.7%) compared to clinicians without its assistance (36.1%) (McNemar's Test: 45.7, p < 0.01) and clinicians with search (44.4%) (4.75, p = 0.03). Further, clinicians assisted by our LLM arrived at more comprehensive differential lists than those without its assistance. Our study suggests that our LLM for DDx has potential to improve clinicians' diagnostic reasoning and accuracy in challenging cases, meriting further real-world evaluation for its ability to empower physicians and widen patients' access to specialist-level expertise.
Predicting Risk of Developing Diabetic Retinopathy using Deep Learning
Aug 10, 2020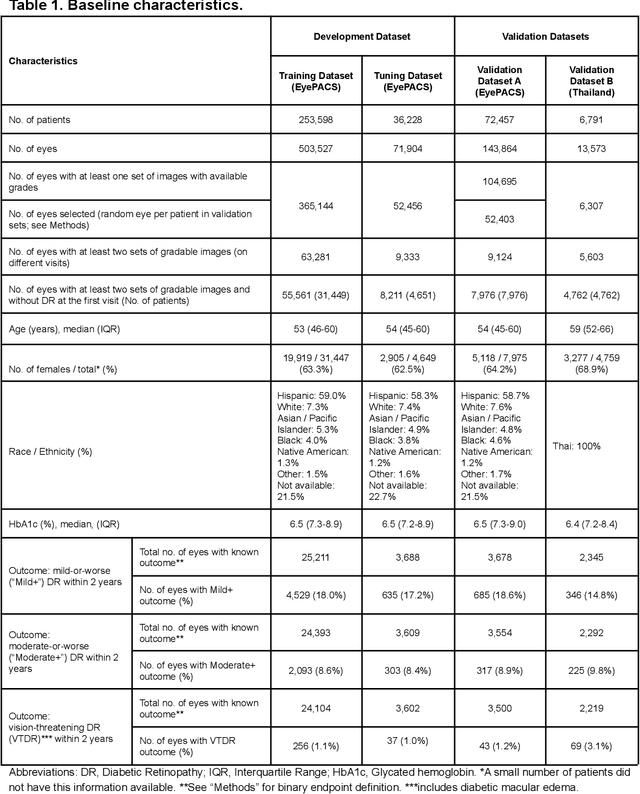

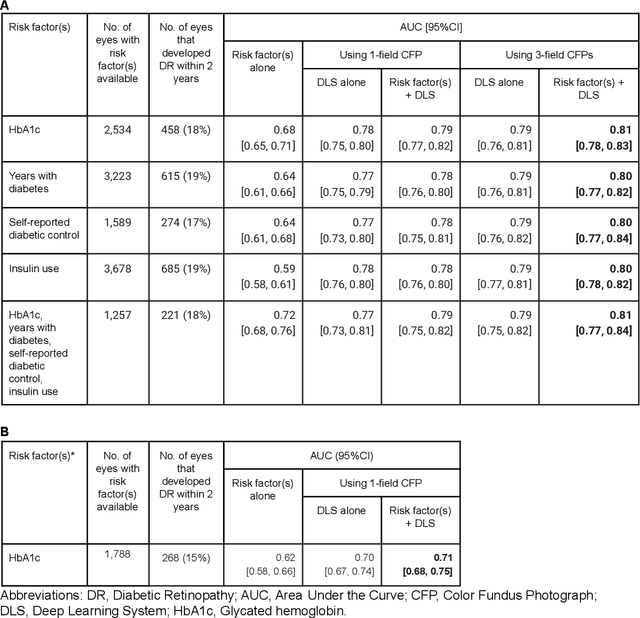

Abstract:Diabetic retinopathy (DR) screening is instrumental in preventing blindness, but faces a scaling challenge as the number of diabetic patients rises. Risk stratification for the development of DR may help optimize screening intervals to reduce costs while improving vision-related outcomes. We created and validated two versions of a deep learning system (DLS) to predict the development of mild-or-worse ("Mild+") DR in diabetic patients undergoing DR screening. The two versions used either three-fields or a single field of color fundus photographs (CFPs) as input. The training set was derived from 575,431 eyes, of which 28,899 had known 2-year outcome, and the remaining were used to augment the training process via multi-task learning. Validation was performed on both an internal validation set (set A; 7,976 eyes; 3,678 with known outcome) and an external validation set (set B; 4,762 eyes; 2,345 with known outcome). For predicting 2-year development of DR, the 3-field DLS had an area under the receiver operating characteristic curve (AUC) of 0.79 (95%CI, 0.78-0.81) on validation set A. On validation set B (which contained only a single field), the 1-field DLS's AUC was 0.70 (95%CI, 0.67-0.74). The DLS was prognostic even after adjusting for available risk factors (p<0.001). When added to the risk factors, the 3-field DLS improved the AUC from 0.72 (95%CI, 0.68-0.76) to 0.81 (95%CI, 0.77-0.84) in validation set A, and the 1-field DLS improved the AUC from 0.62 (95%CI, 0.58-0.66) to 0.71 (95%CI, 0.68-0.75) in validation set B. The DLSs in this study identified prognostic information for DR development from CFPs. This information is independent of and more informative than the available risk factors.
Predicting optical coherence tomography-derived diabetic macular edema grades from fundus photographs using deep learning
Oct 18, 2018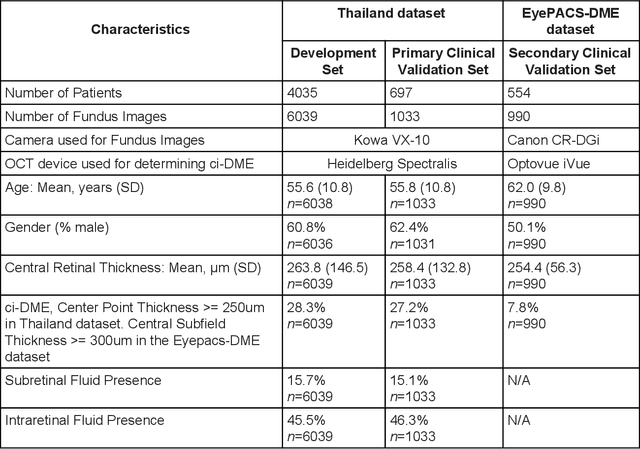
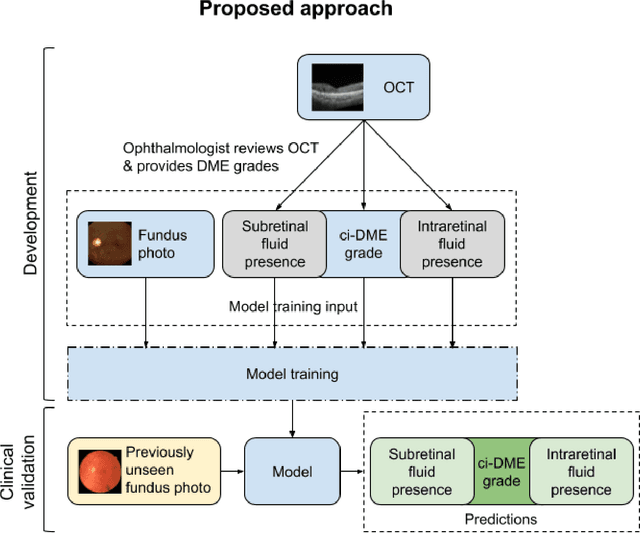
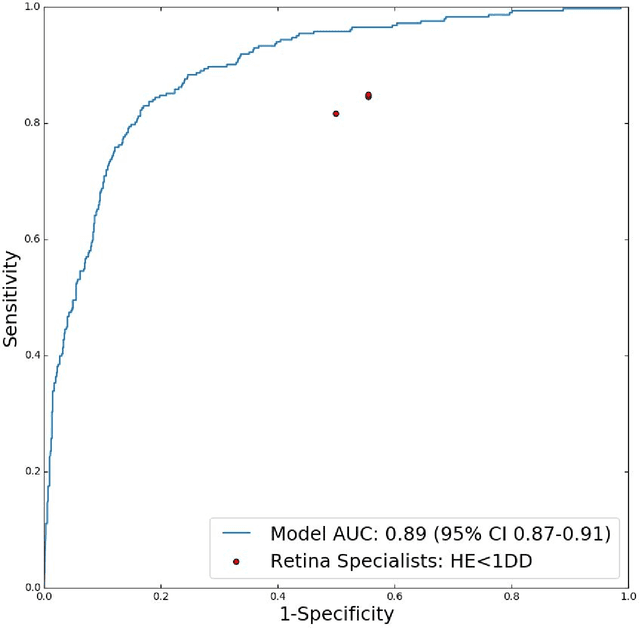
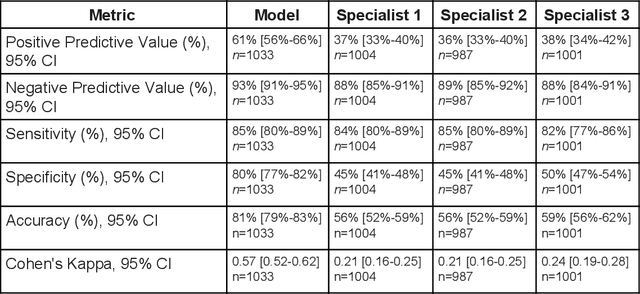
Abstract:Diabetic eye disease is one of the fastest growing causes of preventable blindness. With the advent of anti-VEGF (vascular endothelial growth factor) therapies, it has become increasingly important to detect center-involved diabetic macular edema. However, center-involved diabetic macular edema is diagnosed using optical coherence tomography (OCT), which is not generally available at screening sites because of cost and workflow constraints. Instead, screening programs rely on the detection of hard exudates as a proxy for DME on color fundus photographs, often resulting in high false positive or false negative calls. To improve the accuracy of DME screening, we trained a deep learning model to use color fundus photographs to predict DME grades derived from OCT exams. Our "OCT-DME" model had an AUC of 0.89 (95% CI: 0.87-0.91), which corresponds to a sensitivity of 85% at a specificity of 80%. In comparison, three retinal specialists had similar sensitivities (82-85%), but only half the specificity (45-50%, p<0.001 for each comparison with model). The positive predictive value (PPV) of the OCT-DME model was 61% (95% CI: 56-66%), approximately double the 36-38% by the retina specialists. In addition, we used saliency and other techniques to examine how the model is making its prediction. The ability of deep learning algorithms to make clinically relevant predictions that generally require sophisticated 3D-imaging equipment from simple 2D images has broad relevance to many other applications in medical imaging.
Deep Learning vs. Human Graders for Classifying Severity Levels of Diabetic Retinopathy in a Real-World Nationwide Screening Program
Oct 18, 2018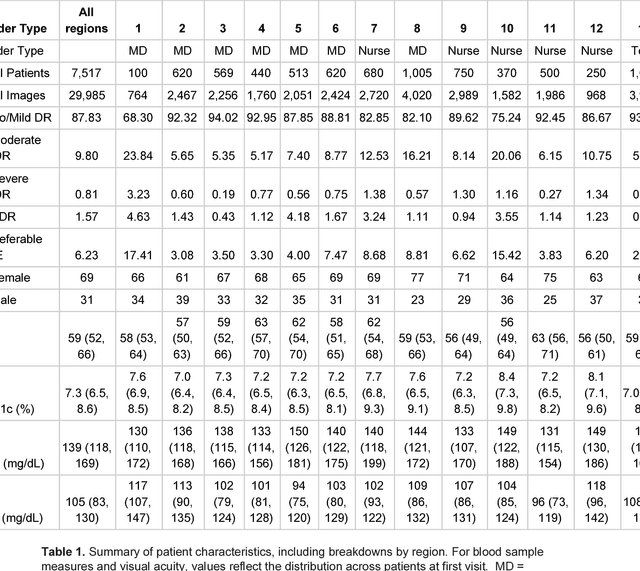
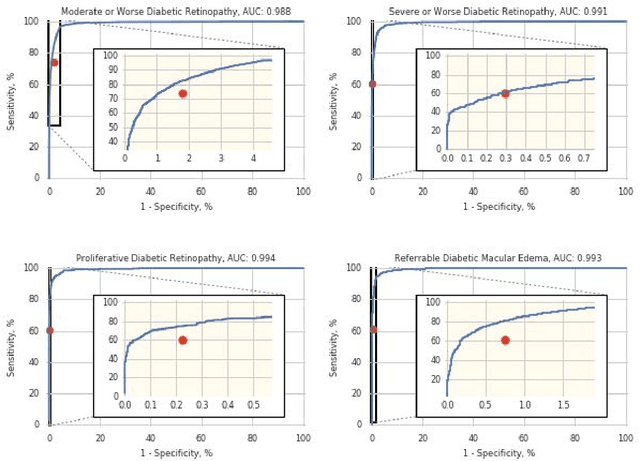
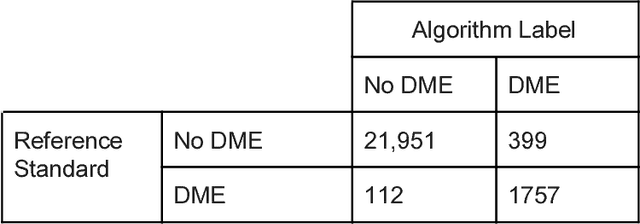
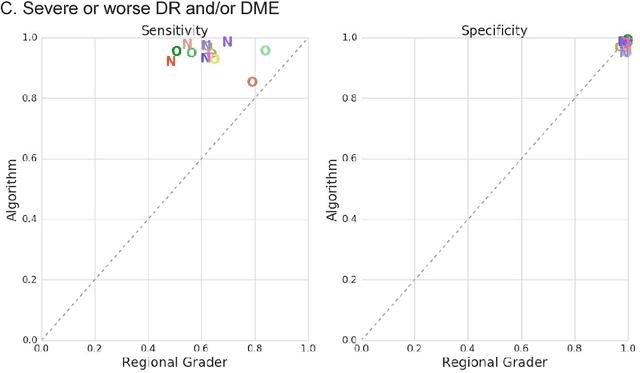
Abstract:Deep learning algorithms have been used to detect diabetic retinopathy (DR) with specialist-level accuracy. This study aims to validate one such algorithm on a large-scale clinical population, and compare the algorithm performance with that of human graders. 25,326 gradable retinal images of patients with diabetes from the community-based, nation-wide screening program of DR in Thailand were analyzed for DR severity and referable diabetic macular edema (DME). Grades adjudicated by a panel of international retinal specialists served as the reference standard. Across different severity levels of DR for determining referable disease, deep learning significantly reduced the false negative rate (by 23%) at the cost of slightly higher false positive rates (2%). Deep learning algorithms may serve as a valuable tool for DR screening.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge