Guilherme de Oliveira Marinho
Predicting Risk of Developing Diabetic Retinopathy using Deep Learning
Aug 10, 2020

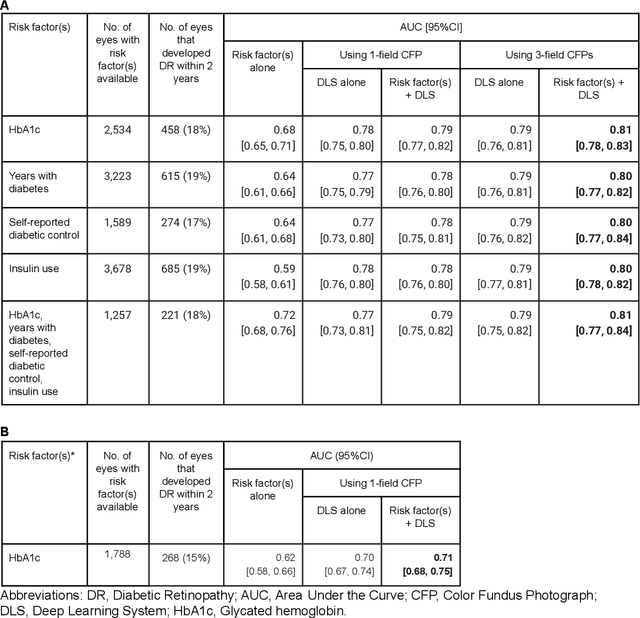

Abstract:Diabetic retinopathy (DR) screening is instrumental in preventing blindness, but faces a scaling challenge as the number of diabetic patients rises. Risk stratification for the development of DR may help optimize screening intervals to reduce costs while improving vision-related outcomes. We created and validated two versions of a deep learning system (DLS) to predict the development of mild-or-worse ("Mild+") DR in diabetic patients undergoing DR screening. The two versions used either three-fields or a single field of color fundus photographs (CFPs) as input. The training set was derived from 575,431 eyes, of which 28,899 had known 2-year outcome, and the remaining were used to augment the training process via multi-task learning. Validation was performed on both an internal validation set (set A; 7,976 eyes; 3,678 with known outcome) and an external validation set (set B; 4,762 eyes; 2,345 with known outcome). For predicting 2-year development of DR, the 3-field DLS had an area under the receiver operating characteristic curve (AUC) of 0.79 (95%CI, 0.78-0.81) on validation set A. On validation set B (which contained only a single field), the 1-field DLS's AUC was 0.70 (95%CI, 0.67-0.74). The DLS was prognostic even after adjusting for available risk factors (p<0.001). When added to the risk factors, the 3-field DLS improved the AUC from 0.72 (95%CI, 0.68-0.76) to 0.81 (95%CI, 0.77-0.84) in validation set A, and the 1-field DLS improved the AUC from 0.62 (95%CI, 0.58-0.66) to 0.71 (95%CI, 0.68-0.75) in validation set B. The DLSs in this study identified prognostic information for DR development from CFPs. This information is independent of and more informative than the available risk factors.
A deep learning system for differential diagnosis of skin diseases
Sep 11, 2019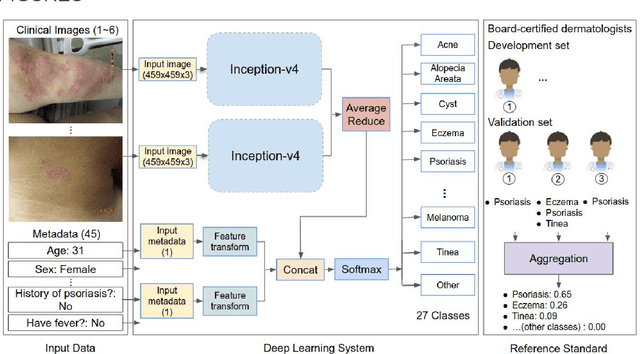
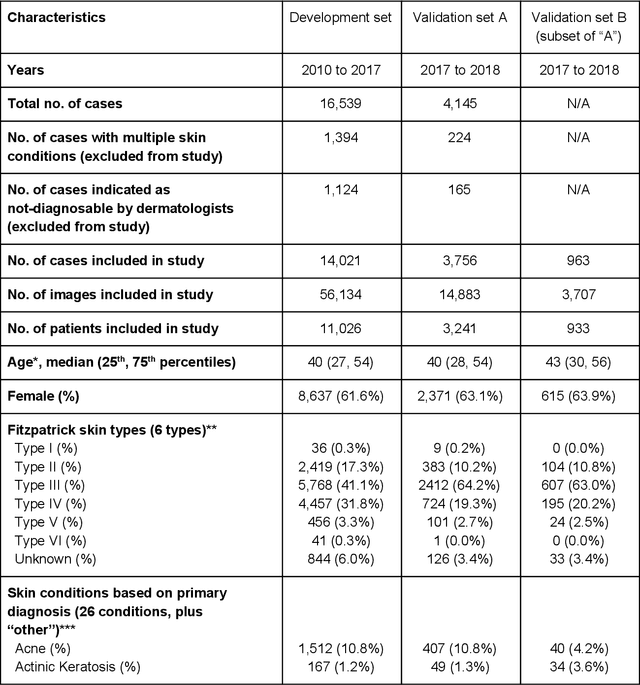
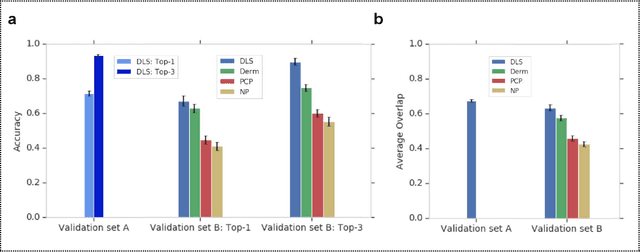
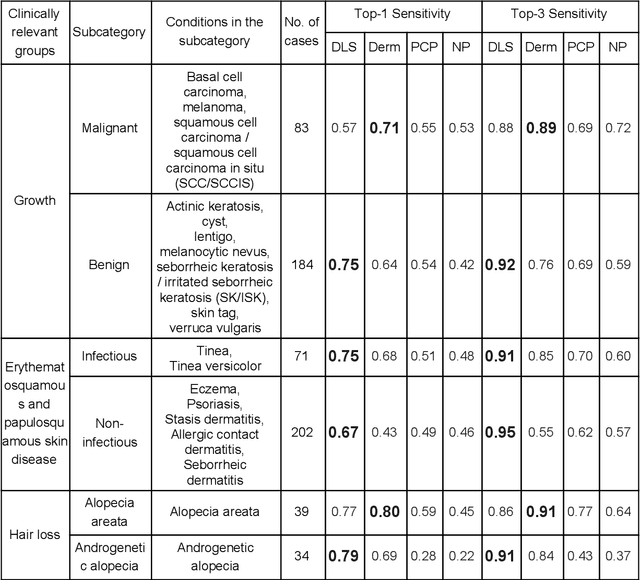
Abstract:Skin conditions affect an estimated 1.9 billion people worldwide. A shortage of dermatologists causes long wait times and leads patients to seek dermatologic care from general practitioners. However, the diagnostic accuracy of general practitioners has been reported to be only 0.24-0.70 (compared to 0.77-0.96 for dermatologists), resulting in referral errors, delays in care, and errors in diagnosis and treatment. In this paper, we developed a deep learning system (DLS) to provide a differential diagnosis of skin conditions for clinical cases (skin photographs and associated medical histories). The DLS distinguishes between 26 skin conditions that represent roughly 80% of the volume of skin conditions seen in primary care. The DLS was developed and validated using de-identified cases from a teledermatology practice serving 17 clinical sites via a temporal split: the first 14,021 cases for development and the last 3,756 cases for validation. On the validation set, where a panel of three board-certified dermatologists defined the reference standard for every case, the DLS achieved 0.71 and 0.93 top-1 and top-3 accuracies respectively. For a random subset of the validation set (n=963 cases), 18 clinicians reviewed the cases for comparison. On this subset, the DLS achieved a 0.67 top-1 accuracy, non-inferior to board-certified dermatologists (0.63, p<0.001), and higher than primary care physicians (PCPs, 0.45) and nurse practitioners (NPs, 0.41). The top-3 accuracy showed a similar trend: 0.90 DLS, 0.75 dermatologists, 0.60 PCPs, and 0.55 NPs. These results highlight the potential of the DLS to augment general practitioners to accurately diagnose skin conditions by suggesting differential diagnoses that may not have been considered. Future work will be needed to prospectively assess the clinical impact of using this tool in actual clinical workflows.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge