Avinash Varadarajan
Training Differentially Private Ad Prediction Models with Semi-Sensitive Features
Jan 26, 2024



Abstract:Motivated by problems arising in digital advertising, we introduce the task of training differentially private (DP) machine learning models with semi-sensitive features. In this setting, a subset of the features is known to the attacker (and thus need not be protected) while the remaining features as well as the label are unknown to the attacker and should be protected by the DP guarantee. This task interpolates between training the model with full DP (where the label and all features should be protected) or with label DP (where all the features are considered known, and only the label should be protected). We present a new algorithm for training DP models with semi-sensitive features. Through an empirical evaluation on real ads datasets, we demonstrate that our algorithm surpasses in utility the baselines of (i) DP stochastic gradient descent (DP-SGD) run on all features (known and unknown), and (ii) a label DP algorithm run only on the known features (while discarding the unknown ones).
Regression with Label Differential Privacy
Dec 12, 2022


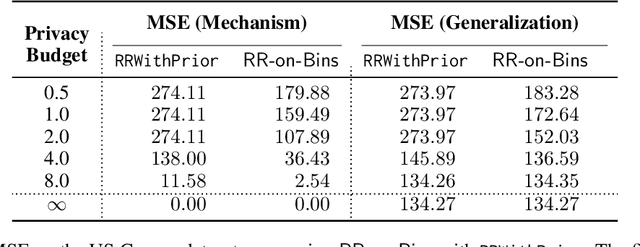
Abstract:We study the task of training regression models with the guarantee of label differential privacy (DP). Based on a global prior distribution on label values, which could be obtained privately, we derive a label DP randomization mechanism that is optimal under a given regression loss function. We prove that the optimal mechanism takes the form of a ``randomized response on bins'', and propose an efficient algorithm for finding the optimal bin values. We carry out a thorough experimental evaluation on several datasets demonstrating the efficacy of our algorithm.
Private Ad Modeling with DP-SGD
Nov 21, 2022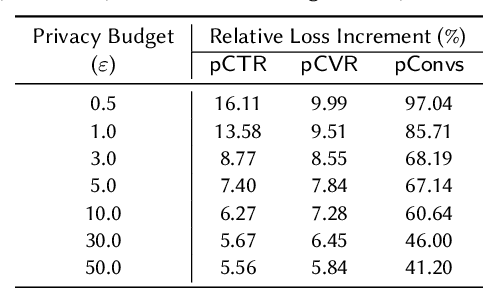
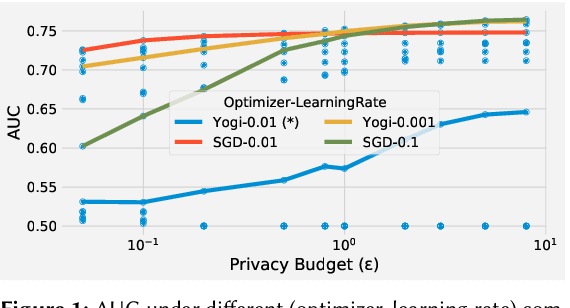
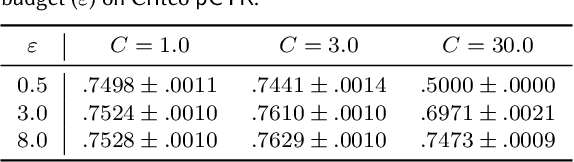
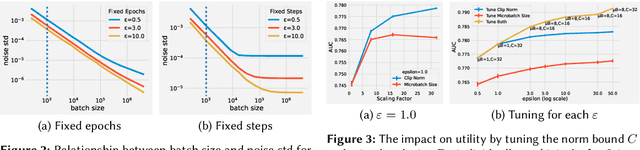
Abstract:A well-known algorithm in privacy-preserving ML is differentially private stochastic gradient descent (DP-SGD). While this algorithm has been evaluated on text and image data, it has not been previously applied to ads data, which are notorious for their high class imbalance and sparse gradient updates. In this work we apply DP-SGD to several ad modeling tasks including predicting click-through rates, conversion rates, and number of conversion events, and evaluate their privacy-utility trade-off on real-world datasets. Our work is the first to empirically demonstrate that DP-SGD can provide both privacy and utility for ad modeling tasks.
Detecting hidden signs of diabetes in external eye photographs
Nov 23, 2020



Abstract:Diabetes-related retinal conditions can be detected by examining the posterior of the eye. By contrast, examining the anterior of the eye can reveal conditions affecting the front of the eye, such as changes to the eyelids, cornea, or crystalline lens. In this work, we studied whether external photographs of the front of the eye can reveal insights into both diabetic retinal diseases and blood glucose control. We developed a deep learning system (DLS) using external eye photographs of 145,832 patients with diabetes from 301 diabetic retinopathy (DR) screening sites in one US state, and evaluated the DLS on three validation sets containing images from 198 sites in 18 other US states. In validation set A (n=27,415 patients, all undilated), the DLS detected poor blood glucose control (HbA1c > 9%) with an area under receiver operating characteristic curve (AUC) of 70.2; moderate-or-worse DR with an AUC of 75.3; diabetic macular edema with an AUC of 78.0; and vision-threatening DR with an AUC of 79.4. For all 4 prediction tasks, the DLS's AUC was higher (p<0.001) than using available self-reported baseline characteristics (age, sex, race/ethnicity, years with diabetes). In terms of positive predictive value, the predicted top 5% of patients had a 67% chance of having HbA1c > 9%, and a 20% chance of having vision threatening diabetic retinopathy. The results generalized to dilated pupils (validation set B, 5,058 patients) and to a different screening service (validation set C, 10,402 patients). Our results indicate that external eye photographs contain information useful for healthcare providers managing patients with diabetes, and may help prioritize patients for in-person screening. Further work is needed to validate these findings on different devices and patient populations (those without diabetes) to evaluate its utility for remote diagnosis and management.
Predicting optical coherence tomography-derived diabetic macular edema grades from fundus photographs using deep learning
Oct 18, 2018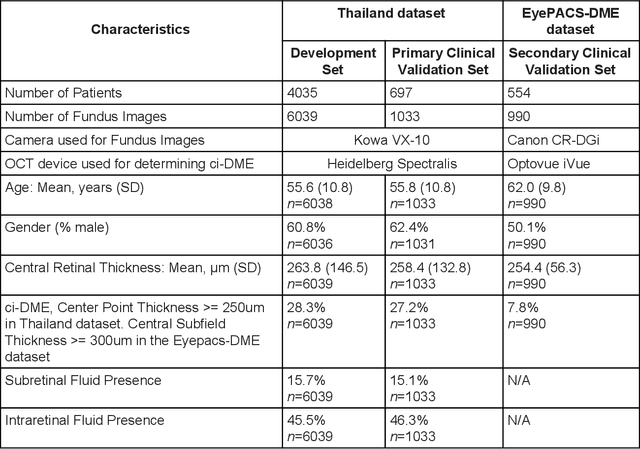
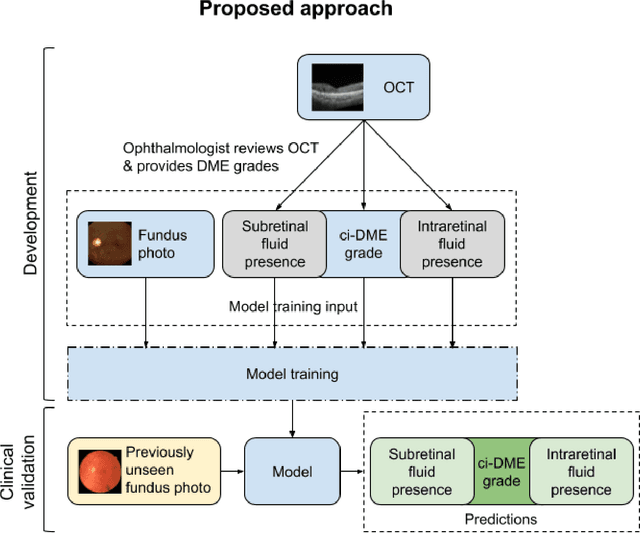
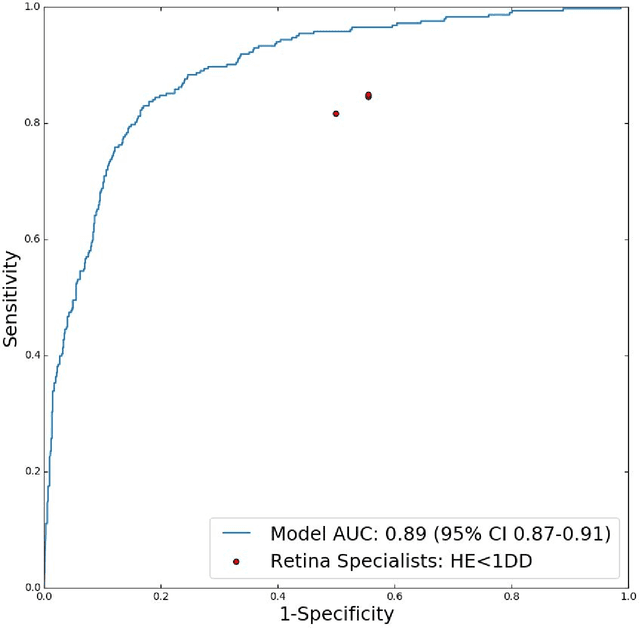
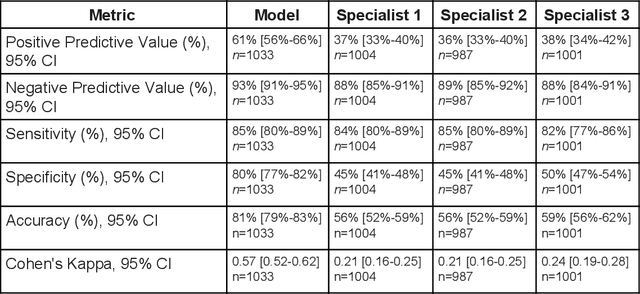
Abstract:Diabetic eye disease is one of the fastest growing causes of preventable blindness. With the advent of anti-VEGF (vascular endothelial growth factor) therapies, it has become increasingly important to detect center-involved diabetic macular edema. However, center-involved diabetic macular edema is diagnosed using optical coherence tomography (OCT), which is not generally available at screening sites because of cost and workflow constraints. Instead, screening programs rely on the detection of hard exudates as a proxy for DME on color fundus photographs, often resulting in high false positive or false negative calls. To improve the accuracy of DME screening, we trained a deep learning model to use color fundus photographs to predict DME grades derived from OCT exams. Our "OCT-DME" model had an AUC of 0.89 (95% CI: 0.87-0.91), which corresponds to a sensitivity of 85% at a specificity of 80%. In comparison, three retinal specialists had similar sensitivities (82-85%), but only half the specificity (45-50%, p<0.001 for each comparison with model). The positive predictive value (PPV) of the OCT-DME model was 61% (95% CI: 56-66%), approximately double the 36-38% by the retina specialists. In addition, we used saliency and other techniques to examine how the model is making its prediction. The ability of deep learning algorithms to make clinically relevant predictions that generally require sophisticated 3D-imaging equipment from simple 2D images has broad relevance to many other applications in medical imaging.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge