Berke Doga Basaran
SegHeD: Segmentation of Heterogeneous Data for Multiple Sclerosis Lesions with Anatomical Constraints
Oct 02, 2024Abstract:Assessment of lesions and their longitudinal progression from brain magnetic resonance (MR) images plays a crucial role in diagnosing and monitoring multiple sclerosis (MS). Machine learning models have demonstrated a great potential for automated MS lesion segmentation. Training such models typically requires large-scale high-quality datasets that are consistently annotated. However, MS imaging datasets are often small, segregated across multiple sites, with different formats (cross-sectional or longitudinal), and diverse annotation styles. This poses a significant challenge to train a unified MS lesion segmentation model. To tackle this challenge, we present SegHeD, a novel multi-dataset multi-task segmentation model that can incorporate heterogeneous data as input and perform all-lesion, new-lesion, as well as vanishing-lesion segmentation. Furthermore, we account for domain knowledge about MS lesions, incorporating longitudinal, spatial, and volumetric constraints into the segmentation model. SegHeD is assessed on five MS datasets and achieves a high performance in all, new, and vanishing-lesion segmentation, outperforming several state-of-the-art methods in this field.
A Foundation Model for Brain Lesion Segmentation with Mixture of Modality Experts
May 16, 2024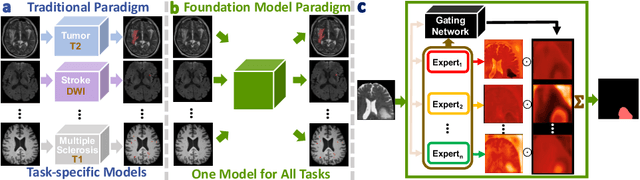
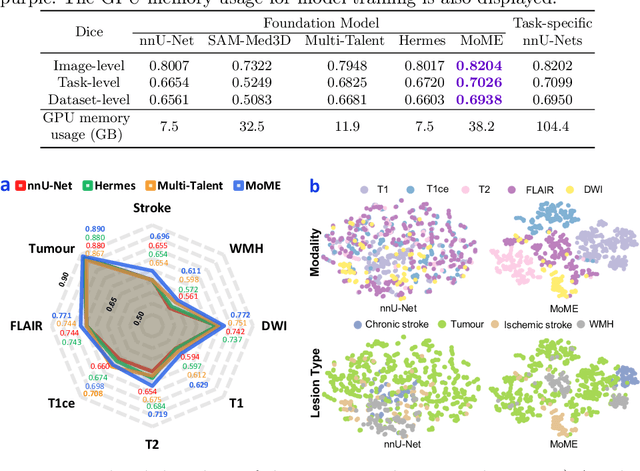

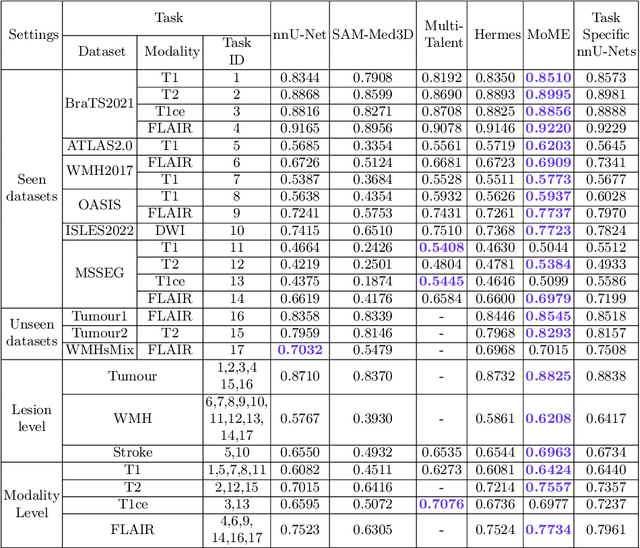
Abstract:Brain lesion segmentation plays an essential role in neurological research and diagnosis. As brain lesions can be caused by various pathological alterations, different types of brain lesions tend to manifest with different characteristics on different imaging modalities. Due to this complexity, brain lesion segmentation methods are often developed in a task-specific manner. A specific segmentation model is developed for a particular lesion type and imaging modality. However, the use of task-specific models requires predetermination of the lesion type and imaging modality, which complicates their deployment in real-world scenarios. In this work, we propose a universal foundation model for 3D brain lesion segmentation, which can automatically segment different types of brain lesions for input data of various imaging modalities. We formulate a novel Mixture of Modality Experts (MoME) framework with multiple expert networks attending to different imaging modalities. A hierarchical gating network combines the expert predictions and fosters expertise collaboration. Furthermore, we introduce a curriculum learning strategy during training to avoid the degeneration of each expert network and preserve their specialization. We evaluated the proposed method on nine brain lesion datasets, encompassing five imaging modalities and eight lesion types. The results show that our model outperforms state-of-the-art universal models and provides promising generalization to unseen datasets.
LesionMix: A Lesion-Level Data Augmentation Method for Medical Image Segmentation
Aug 17, 2023



Abstract:Data augmentation has become a de facto component of deep learning-based medical image segmentation methods. Most data augmentation techniques used in medical imaging focus on spatial and intensity transformations to improve the diversity of training images. They are often designed at the image level, augmenting the full image, and do not pay attention to specific abnormalities within the image. Here, we present LesionMix, a novel and simple lesion-aware data augmentation method. It performs augmentation at the lesion level, increasing the diversity of lesion shape, location, intensity and load distribution, and allowing both lesion populating and inpainting. Experiments on different modalities and different lesion datasets, including four brain MR lesion datasets and one liver CT lesion dataset, demonstrate that LesionMix achieves promising performance in lesion image segmentation, outperforming several recent Mix-based data augmentation methods. The code will be released at https://github.com/dogabasaran/lesionmix.
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
Generative Modelling of the Ageing Heart with Cross-Sectional Imaging and Clinical Data
Aug 28, 2022



Abstract:Cardiovascular disease, the leading cause of death globally, is an age-related disease. Understanding the morphological and functional changes of the heart during ageing is a key scientific question, the answer to which will help us define important risk factors of cardiovascular disease and monitor disease progression. In this work, we propose a novel conditional generative model to describe the changes of 3D anatomy of the heart during ageing. The proposed model is flexible and allows integration of multiple clinical factors (e.g. age, gender) into the generating process. We train the model on a large-scale cross-sectional dataset of cardiac anatomies and evaluate on both cross-sectional and longitudinal datasets. The model demonstrates excellent performance in predicting the longitudinal evolution of the ageing heart and modelling its data distribution.
Subject-Specific Lesion Generation and Pseudo-Healthy Synthesis for Multiple Sclerosis Brain Images
Aug 03, 2022



Abstract:Understanding the intensity characteristics of brain lesions is key for defining image-based biomarkers in neurological studies and for predicting disease burden and outcome. In this work, we present a novel foreground-based generative method for modelling the local lesion characteristics that can both generate synthetic lesions on healthy images and synthesize subject-specific pseudo-healthy images from pathological images. Furthermore, the proposed method can be used as a data augmentation module to generate synthetic images for training brain image segmentation networks. Experiments on multiple sclerosis (MS) brain images acquired on magnetic resonance imaging (MRI) demonstrate that the proposed method can generate highly realistic pseudo-healthy and pseudo-pathological brain images. Data augmentation using the synthetic images improves the brain image segmentation performance compared to traditional data augmentation methods as well as a recent lesion-aware data augmentation technique, CarveMix. The code will be released at https://github.com/dogabasaran/lesion-synthesis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge