Ali Bashashati
AI-driven 3D Spatial Transcriptomics
Feb 25, 2025



Abstract:A comprehensive three-dimensional (3D) map of tissue architecture and gene expression is crucial for illuminating the complexity and heterogeneity of tissues across diverse biomedical applications. However, most spatial transcriptomics (ST) approaches remain limited to two-dimensional (2D) sections of tissue. Although current 3D ST methods hold promise, they typically require extensive tissue sectioning, are complex, are not compatible with non-destructive 3D tissue imaging technologies, and often lack scalability. Here, we present VOlumetrically Resolved Transcriptomics EXpression (VORTEX), an AI framework that leverages 3D tissue morphology and minimal 2D ST to predict volumetric 3D ST. By pretraining on diverse 3D morphology-transcriptomic pairs from heterogeneous tissue samples and then fine-tuning on minimal 2D ST data from a specific volume of interest, VORTEX learns both generic tissue-related and sample-specific morphological correlates of gene expression. This approach enables dense, high-throughput, and fast 3D ST, scaling seamlessly to large tissue volumes far beyond the reach of existing 3D ST techniques. By offering a cost-effective and minimally destructive route to obtaining volumetric molecular insights, we anticipate that VORTEX will accelerate biomarker discovery and our understanding of morphomolecular associations and cell states in complex tissues. Interactive 3D ST volumes can be viewed at https://vortex-demo.github.io/
Benchmarking Histopathology Foundation Models for Ovarian Cancer Bevacizumab Treatment Response Prediction from Whole Slide Images
Jul 30, 2024



Abstract:Bevacizumab is a widely studied targeted therapeutic drug used in conjunction with standard chemotherapy for the treatment of recurrent ovarian cancer. While its administration has shown to increase the progression-free survival (PFS) in patients with advanced stage ovarian cancer, the lack of identifiable biomarkers for predicting patient response has been a major roadblock in its effective adoption towards personalized medicine. In this work, we leverage the latest histopathology foundation models trained on large-scale whole slide image (WSI) datasets to extract ovarian tumor tissue features for predicting bevacizumab response from WSIs. Our extensive experiments across a combination of different histopathology foundation models and multiple instance learning (MIL) strategies demonstrate capability of these large models in predicting bevacizumab response in ovarian cancer patients with the best models achieving an AUC score of 0.86 and an accuracy score of 72.5%. Furthermore, our survival models are able to stratify high- and low-risk cases with statistical significance (p < 0.05) even among the patients with the aggressive subtype of high-grade serous ovarian carcinoma. This work highlights the utility of histopathology foundation models for the task of ovarian bevacizumab response prediction from WSIs. The high-attention regions of the WSIs highlighted by these models not only aid the model explainability but also serve as promising imaging biomarkers for treatment prognosis.
GRASP: GRAph-Structured Pyramidal Whole Slide Image Representation
Feb 06, 2024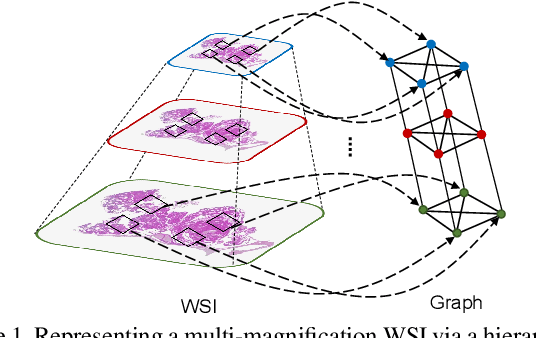
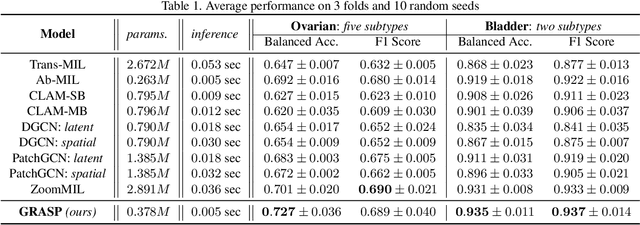
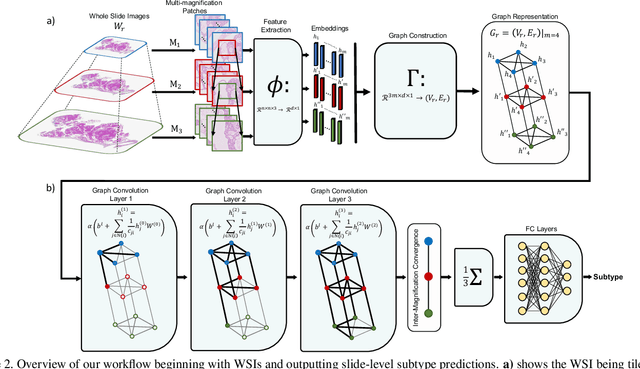
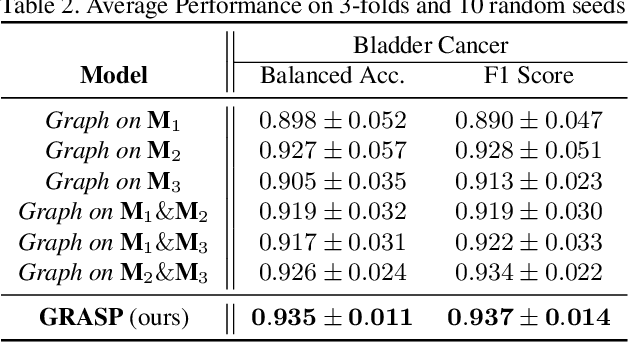
Abstract:Cancer subtyping is one of the most challenging tasks in digital pathology, where Multiple Instance Learning (MIL) by processing gigapixel whole slide images (WSIs) has been in the spotlight of recent research. However, MIL approaches do not take advantage of inter- and intra-magnification information contained in WSIs. In this work, we present GRASP, a novel graph-structured multi-magnification framework for processing WSIs in digital pathology. Our approach is designed to dynamically emulate the pathologist's behavior in handling WSIs and benefits from the hierarchical structure of WSIs. GRASP, which introduces a convergence-based node aggregation instead of traditional pooling mechanisms, outperforms state-of-the-art methods over two distinct cancer datasets by a margin of up to 10% balanced accuracy, while being 7 times smaller than the closest-performing state-of-the-art model in terms of the number of parameters. Our results show that GRASP is dynamic in finding and consulting with different magnifications for subtyping cancers and is reliable and stable across different hyperparameters. The model's behavior has been evaluated by two expert pathologists confirming the interpretability of the model's dynamic. We also provide a theoretical foundation, along with empirical evidence, for our work, explaining how GRASP interacts with different magnifications and nodes in the graph to make predictions. We believe that the strong characteristics yet simple structure of GRASP will encourage the development of interpretable, structure-based designs for WSI representation in digital pathology. Furthermore, we publish two large graph datasets of rare Ovarian and Bladder cancers to contribute to the field.
VOLTA: an Environment-Aware Contrastive Cell Representation Learning for Histopathology
Mar 08, 2023
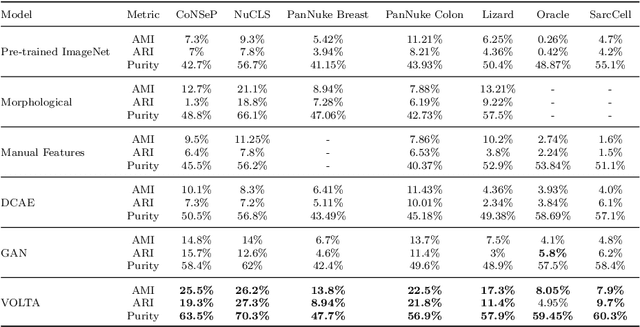

Abstract:In clinical practice, many diagnosis tasks rely on the identification of cells in histopathology images. While supervised machine learning techniques require labels, providing manual cell annotations is time-consuming due to the large number of cells. In this paper, we propose a self-supervised framework (VOLTA) for cell representation learning in histopathology images using a novel technique that accounts for the cell's mutual relationship with its environment for improved cell representations. We subjected our model to extensive experiments on the data collected from multiple institutions around the world comprising of over 700,000 cells, four cancer types, and cell types ranging from three to six categories for each dataset. The results show that our model outperforms the state-of-the-art models in cell representation learning. To showcase the potential power of our proposed framework, we applied VOLTA to ovarian and endometrial cancers with very small sample sizes (10-20 samples) and demonstrated that our cell representations can be utilized to identify the known histotypes of ovarian cancer and provide novel insights that link histopathology and molecular subtypes of endometrial cancer. Unlike supervised deep learning models that require large sample sizes for training, we provide a framework that can empower new discoveries without any annotation data in situations where sample sizes are limited.
AMIGO: Sparse Multi-Modal Graph Transformer with Shared-Context Processing for Representation Learning of Giga-pixel Images
Mar 01, 2023



Abstract:Processing giga-pixel whole slide histopathology images (WSI) is a computationally expensive task. Multiple instance learning (MIL) has become the conventional approach to process WSIs, in which these images are split into smaller patches for further processing. However, MIL-based techniques ignore explicit information about the individual cells within a patch. In this paper, by defining the novel concept of shared-context processing, we designed a multi-modal Graph Transformer (AMIGO) that uses the celluar graph within the tissue to provide a single representation for a patient while taking advantage of the hierarchical structure of the tissue, enabling a dynamic focus between cell-level and tissue-level information. We benchmarked the performance of our model against multiple state-of-the-art methods in survival prediction and showed that ours can significantly outperform all of them including hierarchical Vision Transformer (ViT). More importantly, we show that our model is strongly robust to missing information to an extent that it can achieve the same performance with as low as 20% of the data. Finally, in two different cancer datasets, we demonstrated that our model was able to stratify the patients into low-risk and high-risk groups while other state-of-the-art methods failed to achieve this goal. We also publish a large dataset of immunohistochemistry images (InUIT) containing 1,600 tissue microarray (TMA) cores from 188 patients along with their survival information, making it one of the largest publicly available datasets in this context.
Multi-Scale Relational Graph Convolutional Network for Multiple Instance Learning in Histopathology Images
Dec 17, 2022



Abstract:Graph convolutional neural networks have shown significant potential in natural and histopathology images. However, their use has only been studied in a single magnification or multi-magnification with late fusion. In order to leverage the multi-magnification information and early fusion with graph convolutional networks, we handle different embedding spaces at each magnification by introducing the Multi-Scale Relational Graph Convolutional Network (MS-RGCN) as a multiple instance learning method. We model histopathology image patches and their relation with neighboring patches and patches at other scales (i.e., magnifications) as a graph. To pass the information between different magnification embedding spaces, we define separate message-passing neural networks based on the node and edge type. We experiment on prostate cancer histopathology images to predict the grade groups based on the extracted features from patches. We also compare our MS-RGCN with multiple state-of-the-art methods with evaluations on both source and held-out datasets. Our method outperforms the state-of-the-art on both datasets and especially on the classification of grade groups 2 and 3, which are significant for clinical decisions for patient management. Through an ablation study, we test and show the value of the pertinent design features of the MS-RGCN.
A Morphology Focused Diffusion Probabilistic Model for Synthesis of Histopathology Images
Sep 29, 2022
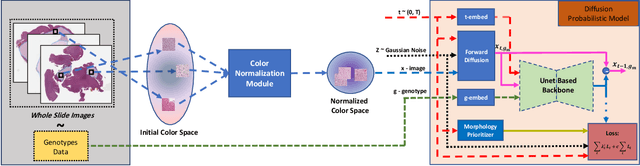
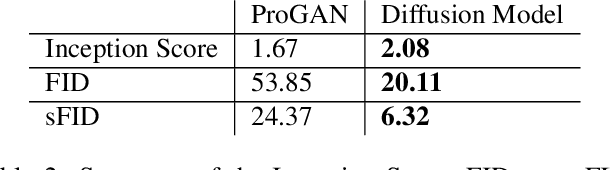
Abstract:Visual microscopic study of diseased tissue by pathologists has been the cornerstone for cancer diagnosis and prognostication for more than a century. Recently, deep learning methods have made significant advances in the analysis and classification of tissue images. However, there has been limited work on the utility of such models in generating histopathology images. These synthetic images have several applications in pathology including utilities in education, proficiency testing, privacy, and data sharing. Recently, diffusion probabilistic models were introduced to generate high quality images. Here, for the first time, we investigate the potential use of such models along with prioritized morphology weighting and color normalization to synthesize high quality histopathology images of brain cancer. Our detailed results show that diffusion probabilistic models are capable of synthesizing a wide range of histopathology images and have superior performance compared to generative adversarial networks.
CCRL: Contrastive Cell Representation Learning
Aug 12, 2022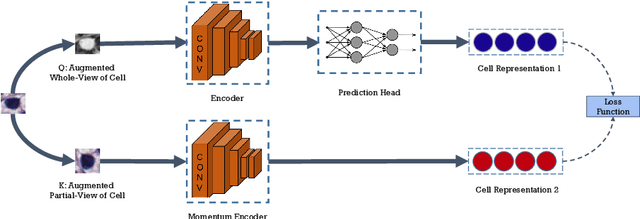
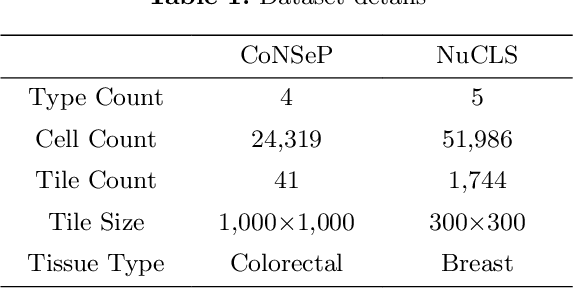
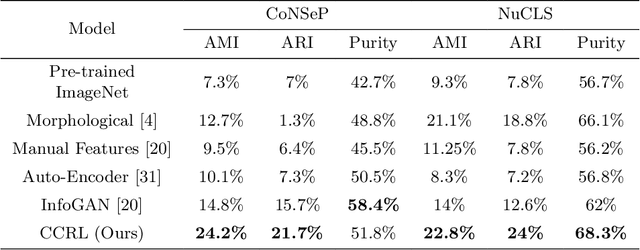
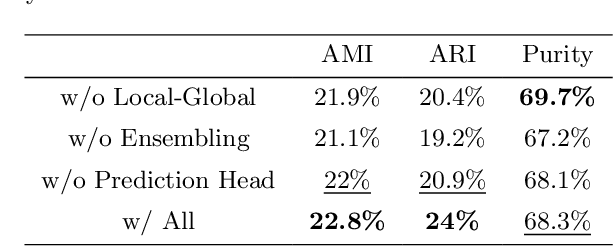
Abstract:Cell identification within the H&E slides is an essential prerequisite that can pave the way towards further pathology analyses including tissue classification, cancer grading, and phenotype prediction. However, performing such a task using deep learning techniques requires a large cell-level annotated dataset. Although previous studies have investigated the performance of contrastive self-supervised methods in tissue classification, the utility of this class of algorithms in cell identification and clustering is still unknown. In this work, we investigated the utility of Self-Supervised Learning (SSL) in cell clustering by proposing the Contrastive Cell Representation Learning (CCRL) model. Through comprehensive comparisons, we show that this model can outperform all currently available cell clustering models by a large margin across two datasets from different tissue types. More interestingly, the results show that our proposed model worked well with a few number of cell categories while the utility of SSL models has been mainly shown in the context of natural image datasets with large numbers of classes (e.g., ImageNet). The unsupervised representation learning approach proposed in this research eliminates the time-consuming step of data annotation in cell classification tasks, which enables us to train our model on a much larger dataset compared to previous methods. Therefore, considering the promising outcome, this approach can open a new avenue to automatic cell representation learning.
Classification of Epithelial Ovarian Carcinoma Whole-Slide Pathology Images Using Deep Transfer Learning
May 22, 2020Abstract:Ovarian cancer is the most lethal cancer of the female reproductive organs. There are $5$ major histological subtypes of epithelial ovarian cancer, each with distinct morphological, genetic, and clinical features. Currently, these histotypes are determined by a pathologist's microscopic examination of tumor whole-slide images (WSI). This process has been hampered by poor inter-observer agreement (Cohen's kappa $0.54$-$0.67$). We utilized a \textit{two}-stage deep transfer learning algorithm based on convolutional neural networks (CNN) and progressive resizing for automatic classification of epithelial ovarian carcinoma WSIs. The proposed algorithm achieved a mean accuracy of $87.54\%$ and Cohen's kappa of $0.8106$ in the slide-level classification of $305$ WSIs; performing better than a standard CNN and pathologists without gynecology-specific training.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge