Yan Su
4D-ACFNet: A 4D Attention Mechanism-Based Prognostic Framework for Colorectal Cancer Liver Metastasis Integrating Multimodal Spatiotemporal Features
Mar 12, 2025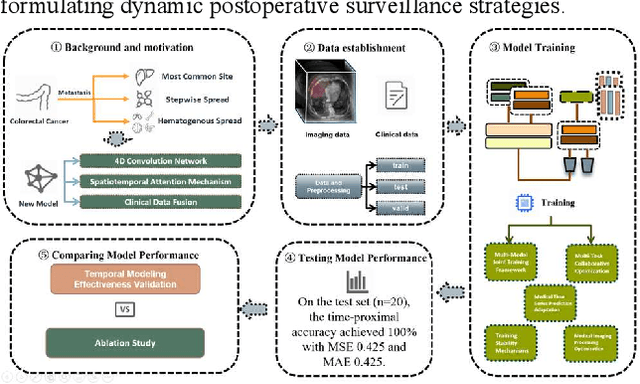

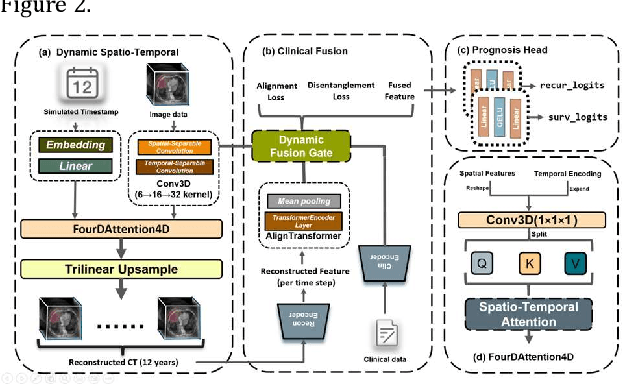

Abstract:Postoperative prognostic prediction for colorectal cancer liver metastasis (CRLM) remains challenging due to tumor heterogeneity, dynamic evolution of the hepatic microenvironment, and insufficient multimodal data fusion. To address these issues, we propose 4D-ACFNet, the first framework that synergistically integrates lightweight spatiotemporal modeling, cross-modal dynamic calibration, and personalized temporal prediction within a unified architecture. Specifically, it incorporates a novel 4D spatiotemporal attention mechanism, which employs spatiotemporal separable convolution (reducing parameter count by 41%) and virtual timestamp encoding to model the interannual evolution patterns of postoperative dynamic processes, such as liver regeneration and steatosis. For cross-modal feature alignment, Transformer layers are integrated to jointly optimize modality alignment loss and disentanglement loss, effectively suppressing scale mismatch and redundant interference in clinical-imaging data. Additionally, we design a dynamic prognostic decision module that generates personalized interannual recurrence risk heatmaps through temporal upsampling and a gated classification head, overcoming the limitations of traditional methods in temporal dynamic modeling and cross-modal alignment. Experiments on 197 CRLM patients demonstrate that the model achieves 100% temporal adjacency accuracy (TAA), with performance significantly surpassing existing approaches. This study establishes the first spatiotemporal modeling paradigm for postoperative dynamic monitoring of CRLM. The proposed framework can be extended to prognostic analysis of multi-cancer metastases, advancing precision surgery from "spatial resection" to "spatiotemporal cure."
RURANET++: An Unsupervised Learning Method for Diabetic Macular Edema Based on SCSE Attention Mechanisms and Dynamic Multi-Projection Head Clustering
Feb 27, 2025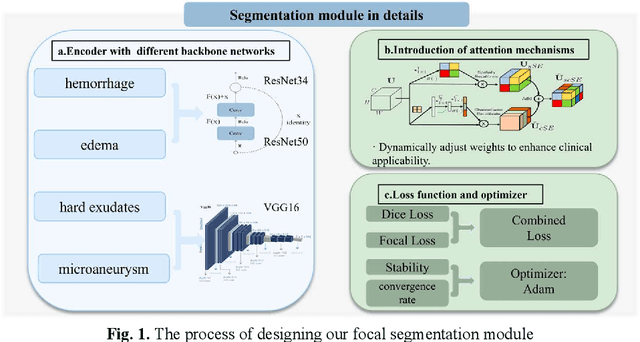
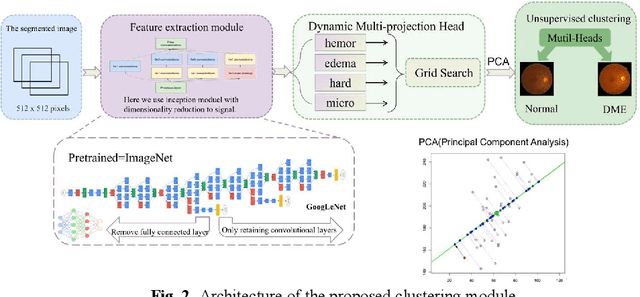
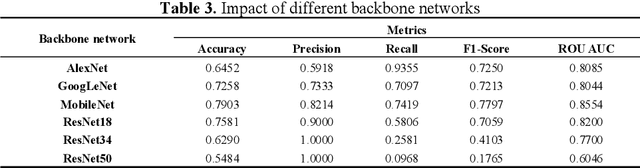
Abstract:Diabetic Macular Edema (DME), a prevalent complication among diabetic patients, constitutes a major cause of visual impairment and blindness. Although deep learning has achieved remarkable progress in medical image analysis, traditional DME diagnosis still relies on extensive annotated data and subjective ophthalmologist assessments, limiting practical applications. To address this, we present RURANET++, an unsupervised learning-based automated DME diagnostic system. This framework incorporates an optimized U-Net architecture with embedded Spatial and Channel Squeeze & Excitation (SCSE) attention mechanisms to enhance lesion feature extraction. During feature processing, a pre-trained GoogLeNet model extracts deep features from retinal images, followed by PCA-based dimensionality reduction to 50 dimensions for computational efficiency. Notably, we introduce a novel clustering algorithm employing multi-projection heads to explicitly control cluster diversity while dynamically adjusting similarity thresholds, thereby optimizing intra-class consistency and inter-class discrimination. Experimental results demonstrate superior performance across multiple metrics, achieving maximum accuracy (0.8411), precision (0.8593), recall (0.8411), and F1-score (0.8390), with exceptional clustering quality. This work provides an efficient unsupervised solution for DME diagnosis with significant clinical implications.
DynSegNet:Dynamic Architecture Adjustment for Adversarial Learning in Segmenting Hemorrhagic Lesions from Fundus Images
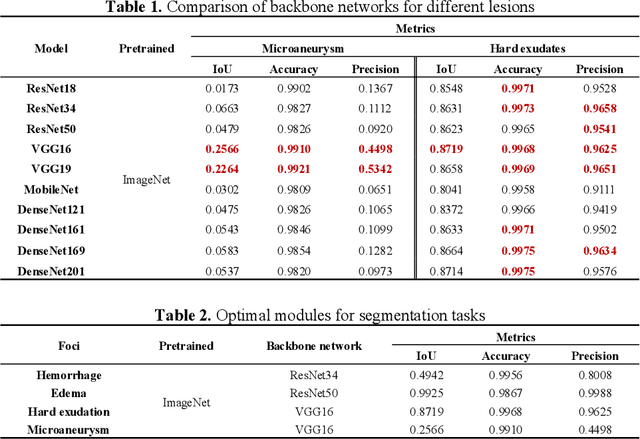
Feb 13, 2025Abstract:The hemorrhagic lesion segmentation plays a critical role in ophthalmic diagnosis, directly influencing early disease detection, treatment planning, and therapeutic efficacy evaluation. However, the task faces significant challenges due to lesion morphological variability, indistinct boundaries, and low contrast with background tissues. To improve diagnostic accuracy and treatment outcomes, developing advanced segmentation techniques remains imperative. This paper proposes an adversarial learning-based dynamic architecture adjustment approach that integrates hierarchical U-shaped encoder-decoder, residual blocks, attention mechanisms, and ASPP modules. By dynamically optimizing feature fusion, our method enhances segmentation performance. Experimental results demonstrate a Dice coefficient of 0.6802, IoU of 0.5602, Recall of 0.766, Precision of 0.6525, and Accuracy of 0.9955, effectively addressing the challenges in fundus image hemorrhage segmentation.[* Corresponding author.]
Investigating the Capabilities of Deep Learning for Processing and Interpreting One-Shot Multi-offset GPR Data: A Numerical Case Study for Lunar and Martian Environments
Oct 18, 2024



Abstract:Ground-penetrating radar (GPR) is a mature geophysical method that has gained increasing popularity in planetary science over the past decade. GPR has been utilised both for Lunar and Martian missions providing pivotal information regarding the near surface geology of Terrestrial planets. Within that context, numerous processing pipelines have been suggested to address the unique challenges present in planetary setups. These processing pipelines often require manual tuning resulting to ambiguous outputs open to non-unique interpretations. These pitfalls combined with the large number of planetary GPR data (kilometers in magnitude), highlight the necessity for automatic, objective and advanced processing and interpretation schemes. The current paper investigates the potential of deep learning for interpreting and processing GPR data. The one-shot multi-offset configuration is investigated via a coherent numerical case study, showcasing the potential of deep learning for A) reconstructing the dielectric distribution of the the near surface of Terrestrial planets, and B) filling missing or bad-quality traces. Special care was taken for the numerical data to be both realistic and challenging. Moreover, the generated synthetic data are properly labelled and made publicly available for training future data-driven pipelines and contributing towards developing pre-trained foundation models for GPR.
Deep learning for in vitro prediction of pharmaceutical formulations
Sep 06, 2018
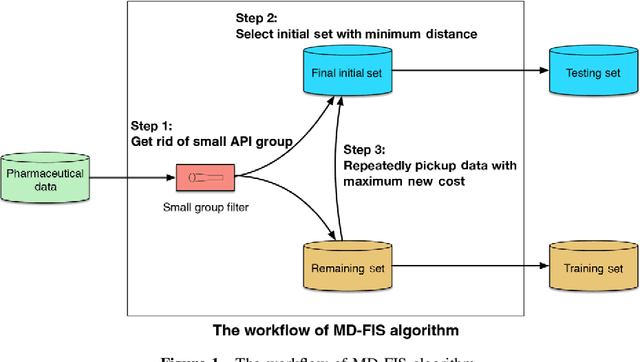

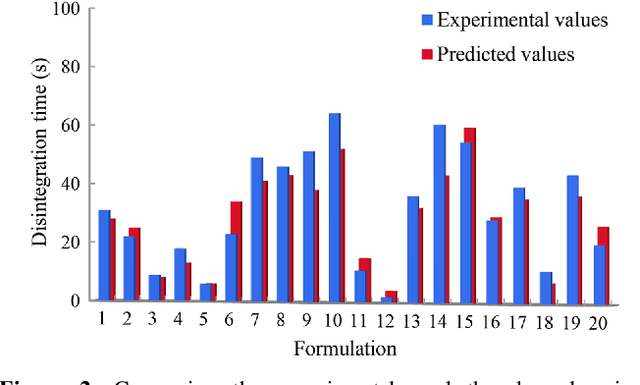
Abstract:Current pharmaceutical formulation development still strongly relies on the traditional trial-and-error approach by individual experiences of pharmaceutical scientists, which is laborious, time-consuming and costly. Recently, deep learning has been widely applied in many challenging domains because of its important capability of automatic feature extraction. The aim of this research is to use deep learning to predict pharmaceutical formulations. In this paper, two different types of dosage forms were chosen as model systems. Evaluation criteria suitable for pharmaceutics were applied to assessing the performance of the models. Moreover, an automatic dataset selection algorithm was developed for selecting the representative data as validation and test datasets. Six machine learning methods were compared with deep learning. The result shows the accuracies of both two deep neural networks were above 80% and higher than other machine learning models, which showed good prediction in pharmaceutical formulations. In summary, deep learning with the automatic data splitting algorithm and the evaluation criteria suitable for pharmaceutical formulation data was firstly developed for the prediction of pharmaceutical formulations. The cross-disciplinary integration of pharmaceutics and artificial intelligence may shift the paradigm of pharmaceutical researches from experience-dependent studies to data-driven methodologies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge