Zhuyifan Ye
An Integrated Transfer Learning and Multitask Learning Approach for Pharmacokinetic Parameter Prediction
Dec 21, 2018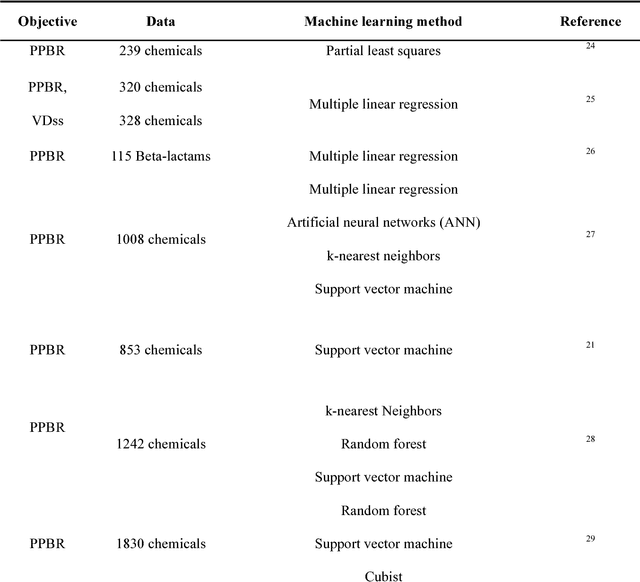
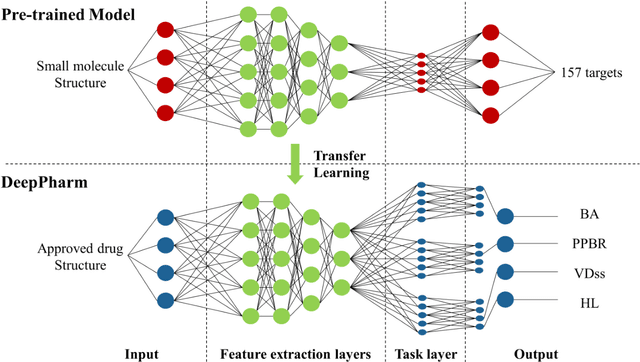
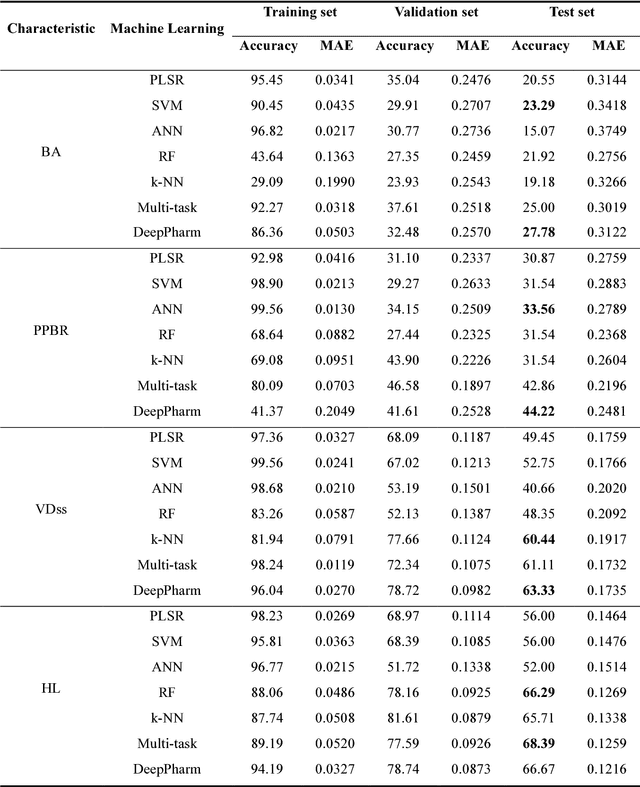

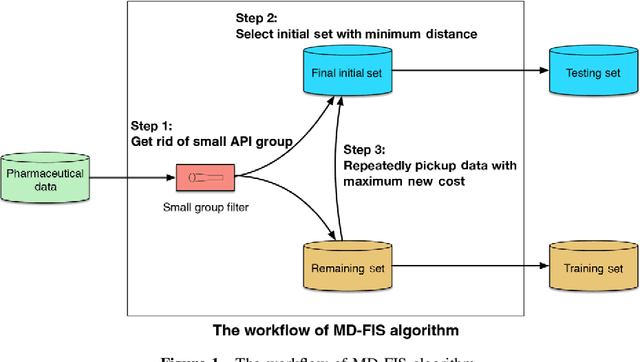
Abstract:Background: Pharmacokinetic evaluation is one of the key processes in drug discovery and development. However, current absorption, distribution, metabolism, excretion prediction models still have limited accuracy. Aim: This study aims to construct an integrated transfer learning and multitask learning approach for developing quantitative structure-activity relationship models to predict four human pharmacokinetic parameters. Methods: A pharmacokinetic dataset included 1104 U.S. FDA approved small molecule drugs. The dataset included four human pharmacokinetic parameter subsets (oral bioavailability, plasma protein binding rate, apparent volume of distribution at steady-state and elimination half-life). The pre-trained model was trained on over 30 million bioactivity data. An integrated transfer learning and multitask learning approach was established to enhance the model generalization. Results: The pharmacokinetic dataset was split into three parts (60:20:20) for training, validation and test by the improved Maximum Dissimilarity algorithm with the representative initial set selection algorithm and the weighted distance function. The multitask learning techniques enhanced the model predictive ability. The integrated transfer learning and multitask learning model demonstrated the best accuracies, because deep neural networks have the general feature extraction ability, transfer learning and multitask learning improved the model generalization. Conclusions: The integrated transfer learning and multitask learning approach with the improved dataset splitting algorithm was firstly introduced to predict the pharmacokinetic parameters. This method can be further employed in drug discovery and development.
Deep learning for in vitro prediction of pharmaceutical formulations
Sep 06, 2018

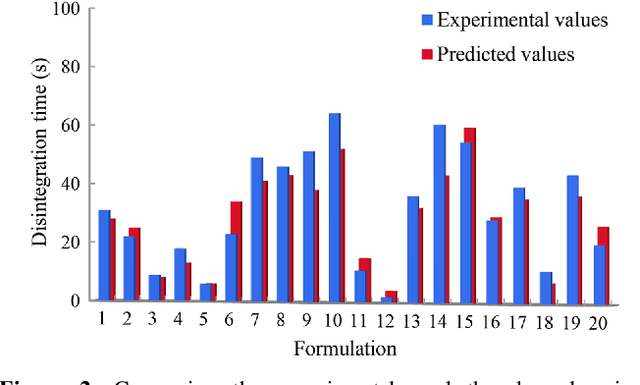
Abstract:Current pharmaceutical formulation development still strongly relies on the traditional trial-and-error approach by individual experiences of pharmaceutical scientists, which is laborious, time-consuming and costly. Recently, deep learning has been widely applied in many challenging domains because of its important capability of automatic feature extraction. The aim of this research is to use deep learning to predict pharmaceutical formulations. In this paper, two different types of dosage forms were chosen as model systems. Evaluation criteria suitable for pharmaceutics were applied to assessing the performance of the models. Moreover, an automatic dataset selection algorithm was developed for selecting the representative data as validation and test datasets. Six machine learning methods were compared with deep learning. The result shows the accuracies of both two deep neural networks were above 80% and higher than other machine learning models, which showed good prediction in pharmaceutical formulations. In summary, deep learning with the automatic data splitting algorithm and the evaluation criteria suitable for pharmaceutical formulation data was firstly developed for the prediction of pharmaceutical formulations. The cross-disciplinary integration of pharmaceutics and artificial intelligence may shift the paradigm of pharmaceutical researches from experience-dependent studies to data-driven methodologies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge