Zesheng Li
RecurGS: Interactive Scene Modeling via Discrete-State Recurrent Gaussian Fusion
Dec 20, 2025Abstract:Recent advances in 3D scene representations have enabled high-fidelity novel view synthesis, yet adapting to discrete scene changes and constructing interactive 3D environments remain open challenges in vision and robotics. Existing approaches focus solely on updating a single scene without supporting novel-state synthesis. Others rely on diffusion-based object-background decoupling that works on one state at a time and cannot fuse information across multiple observations. To address these limitations, we introduce RecurGS, a recurrent fusion framework that incrementally integrates discrete Gaussian scene states into a single evolving representation capable of interaction. RecurGS detects object-level changes across consecutive states, aligns their geometric motion using semantic correspondence and Lie-algebra based SE(3) refinement, and performs recurrent updates that preserve historical structures through replay supervision. A voxelized, visibility-aware fusion module selectively incorporates newly observed regions while keeping stable areas fixed, mitigating catastrophic forgetting and enabling efficient long-horizon updates. RecurGS supports object-level manipulation, synthesizes novel scene states without requiring additional scans, and maintains photorealistic fidelity across evolving environments. Extensive experiments across synthetic and real-world datasets demonstrate that our framework delivers high-quality reconstructions with substantially improved update efficiency, providing a scalable step toward continuously interactive Gaussian worlds.
Multi-level Dynamic Style Transfer for NeRFs
Oct 01, 2025



Abstract:As the application of neural radiance fields (NeRFs) in various 3D vision tasks continues to expand, numerous NeRF-based style transfer techniques have been developed. However, existing methods typically integrate style statistics into the original NeRF pipeline, often leading to suboptimal results in both content preservation and artistic stylization. In this paper, we present multi-level dynamic style transfer for NeRFs (MDS-NeRF), a novel approach that reengineers the NeRF pipeline specifically for stylization and incorporates an innovative dynamic style injection module. Particularly, we propose a multi-level feature adaptor that helps generate a multi-level feature grid representation from the content radiance field, effectively capturing the multi-scale spatial structure of the scene. In addition, we present a dynamic style injection module that learns to extract relevant style features and adaptively integrates them into the content patterns. The stylized multi-level features are then transformed into the final stylized view through our proposed multi-level cascade decoder. Furthermore, we extend our 3D style transfer method to support omni-view style transfer using 3D style references. Extensive experiments demonstrate that MDS-NeRF achieves outstanding performance for 3D style transfer, preserving multi-scale spatial structures while effectively transferring stylistic characteristics.
IGFuse: Interactive 3D Gaussian Scene Reconstruction via Multi-Scans Fusion
Aug 18, 2025



Abstract:Reconstructing complete and interactive 3D scenes remains a fundamental challenge in computer vision and robotics, particularly due to persistent object occlusions and limited sensor coverage. Multiview observations from a single scene scan often fail to capture the full structural details. Existing approaches typically rely on multi stage pipelines, such as segmentation, background completion, and inpainting or require per-object dense scanning, both of which are error-prone, and not easily scalable. We propose IGFuse, a novel framework that reconstructs interactive Gaussian scene by fusing observations from multiple scans, where natural object rearrangement between captures reveal previously occluded regions. Our method constructs segmentation aware Gaussian fields and enforces bi-directional photometric and semantic consistency across scans. To handle spatial misalignments, we introduce a pseudo-intermediate scene state for unified alignment, alongside collaborative co-pruning strategies to refine geometry. IGFuse enables high fidelity rendering and object level scene manipulation without dense observations or complex pipelines. Extensive experiments validate the framework's strong generalization to novel scene configurations, demonstrating its effectiveness for real world 3D reconstruction and real-to-simulation transfer. Our project page is available online.
STG: Spatiotemporal Graph Neural Network with Fusion and Spatiotemporal Decoupling Learning for Prognostic Prediction of Colorectal Cancer Liver Metastasis
May 06, 2025Abstract:We propose a multimodal spatiotemporal graph neural network (STG) framework to predict colorectal cancer liver metastasis (CRLM) progression. Current clinical models do not effectively integrate the tumor's spatial heterogeneity, dynamic evolution, and complex multimodal data relationships, limiting their predictive accuracy. Our STG framework combines preoperative CT imaging and clinical data into a heterogeneous graph structure, enabling joint modeling of tumor distribution and temporal evolution through spatial topology and cross-modal edges. The framework uses GraphSAGE to aggregate spatiotemporal neighborhood information and leverages supervised and contrastive learning strategies to enhance the model's ability to capture temporal features and improve robustness. A lightweight version of the model reduces parameter count by 78.55%, maintaining near-state-of-the-art performance. The model jointly optimizes recurrence risk regression and survival analysis tasks, with contrastive loss improving feature representational discriminability and cross-modal consistency. Experimental results on the MSKCC CRLM dataset show a time-adjacent accuracy of 85% and a mean absolute error of 1.1005, significantly outperforming existing methods. The innovative heterogeneous graph construction and spatiotemporal decoupling mechanism effectively uncover the associations between dynamic tumor microenvironment changes and prognosis, providing reliable quantitative support for personalized treatment decisions.
4D-ACFNet: A 4D Attention Mechanism-Based Prognostic Framework for Colorectal Cancer Liver Metastasis Integrating Multimodal Spatiotemporal Features
Mar 12, 2025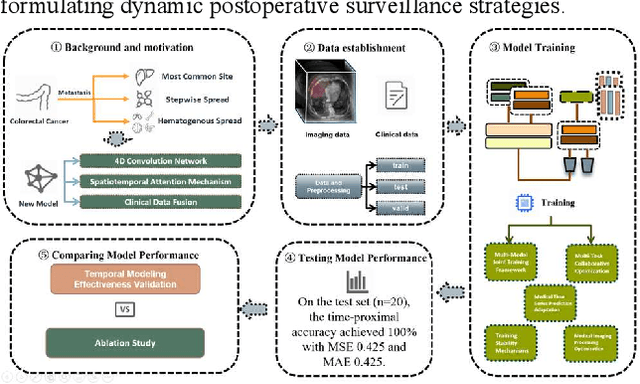

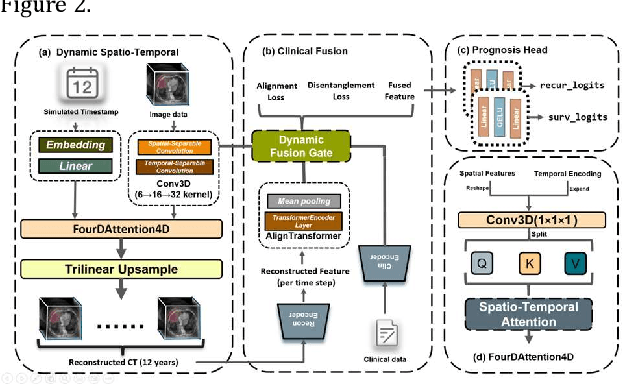

Abstract:Postoperative prognostic prediction for colorectal cancer liver metastasis (CRLM) remains challenging due to tumor heterogeneity, dynamic evolution of the hepatic microenvironment, and insufficient multimodal data fusion. To address these issues, we propose 4D-ACFNet, the first framework that synergistically integrates lightweight spatiotemporal modeling, cross-modal dynamic calibration, and personalized temporal prediction within a unified architecture. Specifically, it incorporates a novel 4D spatiotemporal attention mechanism, which employs spatiotemporal separable convolution (reducing parameter count by 41%) and virtual timestamp encoding to model the interannual evolution patterns of postoperative dynamic processes, such as liver regeneration and steatosis. For cross-modal feature alignment, Transformer layers are integrated to jointly optimize modality alignment loss and disentanglement loss, effectively suppressing scale mismatch and redundant interference in clinical-imaging data. Additionally, we design a dynamic prognostic decision module that generates personalized interannual recurrence risk heatmaps through temporal upsampling and a gated classification head, overcoming the limitations of traditional methods in temporal dynamic modeling and cross-modal alignment. Experiments on 197 CRLM patients demonstrate that the model achieves 100% temporal adjacency accuracy (TAA), with performance significantly surpassing existing approaches. This study establishes the first spatiotemporal modeling paradigm for postoperative dynamic monitoring of CRLM. The proposed framework can be extended to prognostic analysis of multi-cancer metastases, advancing precision surgery from "spatial resection" to "spatiotemporal cure."
DynSegNet:Dynamic Architecture Adjustment for Adversarial Learning in Segmenting Hemorrhagic Lesions from Fundus Images
Feb 13, 2025Abstract:The hemorrhagic lesion segmentation plays a critical role in ophthalmic diagnosis, directly influencing early disease detection, treatment planning, and therapeutic efficacy evaluation. However, the task faces significant challenges due to lesion morphological variability, indistinct boundaries, and low contrast with background tissues. To improve diagnostic accuracy and treatment outcomes, developing advanced segmentation techniques remains imperative. This paper proposes an adversarial learning-based dynamic architecture adjustment approach that integrates hierarchical U-shaped encoder-decoder, residual blocks, attention mechanisms, and ASPP modules. By dynamically optimizing feature fusion, our method enhances segmentation performance. Experimental results demonstrate a Dice coefficient of 0.6802, IoU of 0.5602, Recall of 0.766, Precision of 0.6525, and Accuracy of 0.9955, effectively addressing the challenges in fundus image hemorrhage segmentation.[* Corresponding author.]
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge