Qianjun Jia
Multi-Cycle-Consistent Adversarial Networks for Edge Denoising of Computed Tomography Images
Apr 25, 2021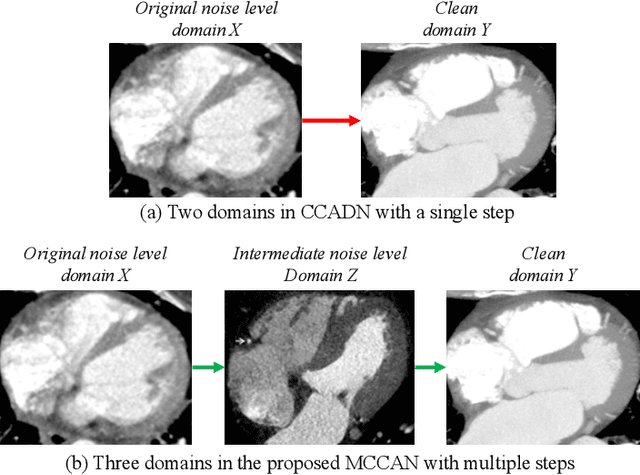
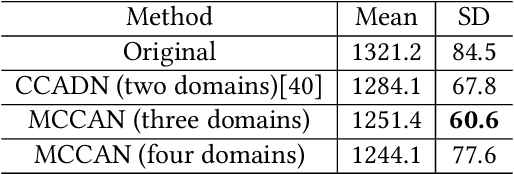
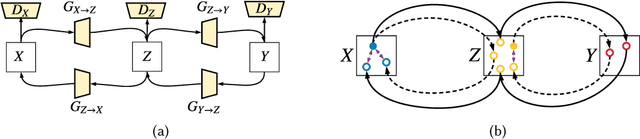
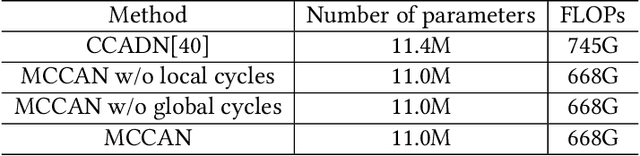
Abstract:As one of the most commonly ordered imaging tests, computed tomography (CT) scan comes with inevitable radiation exposure that increases the cancer risk to patients. However, CT image quality is directly related to radiation dose, thus it is desirable to obtain high-quality CT images with as little dose as possible. CT image denoising tries to obtain high dose like high-quality CT images (domain X) from low dose low-quality CTimages (domain Y), which can be treated as an image-to-image translation task where the goal is to learn the transform between a source domain X (noisy images) and a target domain Y (clean images). In this paper, we propose a multi-cycle-consistent adversarial network (MCCAN) that builds intermediate domains and enforces both local and global cycle-consistency for edge denoising of CT images. The global cycle-consistency couples all generators together to model the whole denoising process, while the local cycle-consistency imposes effective supervision on the process between adjacent domains. Experiments show that both local and global cycle-consistency are important for the success of MCCAN, which outperformsCCADN in terms of denoising quality with slightly less computation resource consumption.
ImageCHD: A 3D Computed Tomography Image Dataset for Classification of Congenital Heart Disease
Jan 26, 2021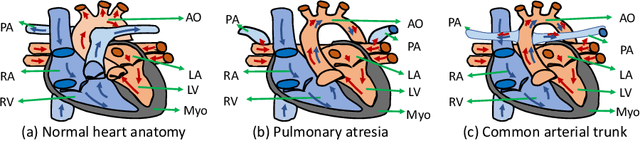
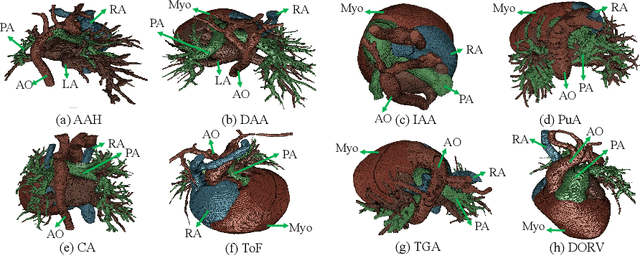
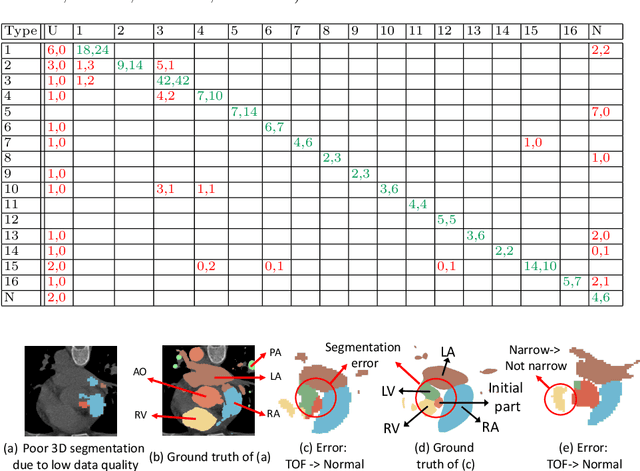
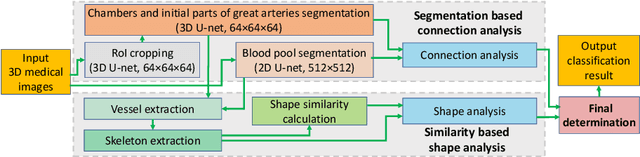
Abstract:Congenital heart disease (CHD) is the most common type of birth defect, which occurs 1 in every 110 births in the United States. CHD usually comes with severe variations in heart structure and great artery connections that can be classified into many types. Thus highly specialized domain knowledge and the time-consuming human process is needed to analyze the associated medical images. On the other hand, due to the complexity of CHD and the lack of dataset, little has been explored on the automatic diagnosis (classification) of CHDs. In this paper, we present ImageCHD, the first medical image dataset for CHD classification. ImageCHD contains 110 3D Computed Tomography (CT) images covering most types of CHD, which is of decent size Classification of CHDs requires the identification of large structural changes without any local tissue changes, with limited data. It is an example of a larger class of problems that are quite difficult for current machine-learning-based vision methods to solve. To demonstrate this, we further present a baseline framework for the automatic classification of CHD, based on a state-of-the-art CHD segmentation method. Experimental results show that the baseline framework can only achieve a classification accuracy of 82.0\% under a selective prediction scheme with 88.4\% coverage, leaving big room for further improvement. We hope that ImageCHD can stimulate further research and lead to innovative and generic solutions that would have an impact in multiple domains. Our dataset is released to the public compared with existing medical imaging datasets.
Myocardial Segmentation of Cardiac MRI Sequences with Temporal Consistency for Coronary Artery Disease Diagnosis
Dec 29, 2020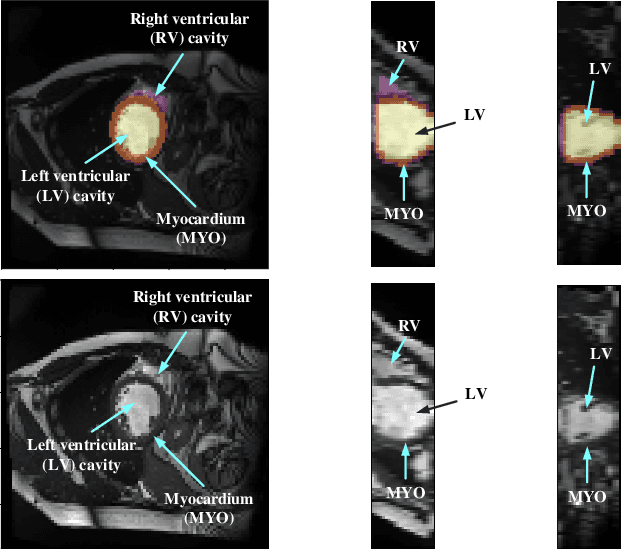
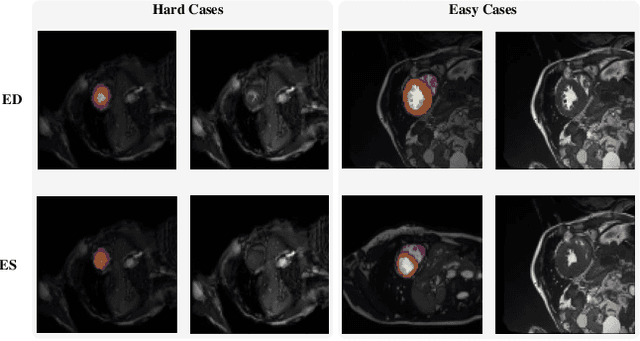
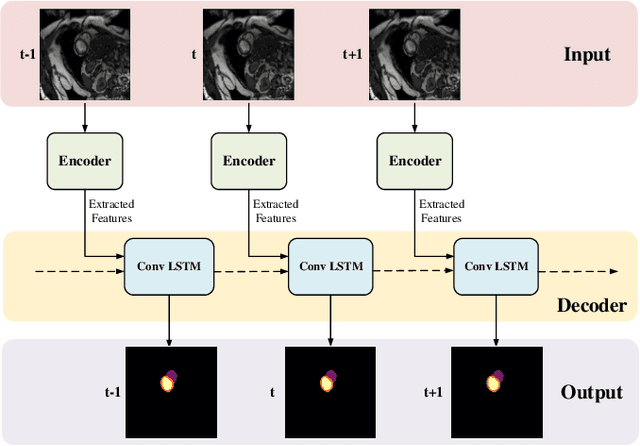
Abstract:Coronary artery disease (CAD) is the most common cause of death globally, and its diagnosis is usually based on manual myocardial segmentation of Magnetic Resonance Imaging (MRI) sequences. As the manual segmentation is tedious, time-consuming and with low applicability, automatic myocardial segmentation using machine learning techniques has been widely explored recently. However, almost all the existing methods treat the input MRI sequences independently, which fails to capture the temporal information between sequences, e.g., the shape and location information of the myocardium in sequences along time. In this paper, we propose a myocardial segmentation framework for sequence of cardiac MRI (CMR) scanning images of left ventricular cavity, right ventricular cavity, and myocardium. Specifically, we propose to combine conventional networks and recurrent networks to incorporate temporal information between sequences to ensure temporal consistent. We evaluated our framework on the Automated Cardiac Diagnosis Challenge (ACDC) dataset. Experiment results demonstrate that our framework can improve the segmentation accuracy by up to 2% in Dice coefficient.
Do Noises Bother Human and Neural Networks In the Same Way? A Medical Image Analysis Perspective
Nov 04, 2020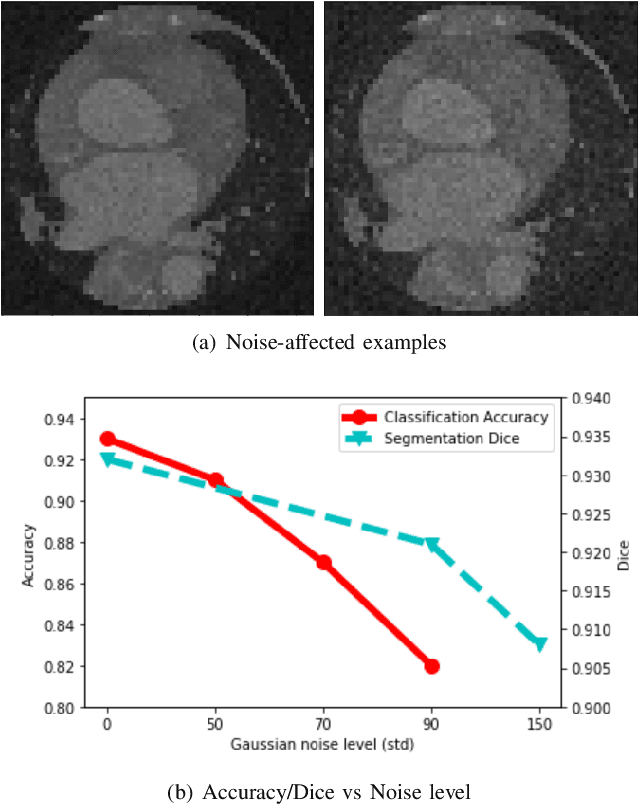
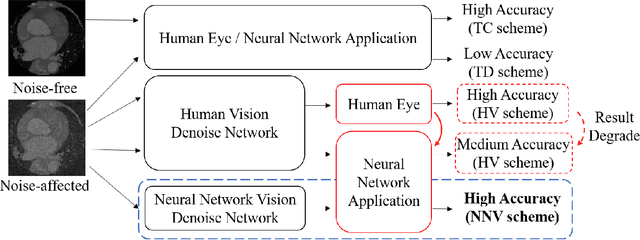
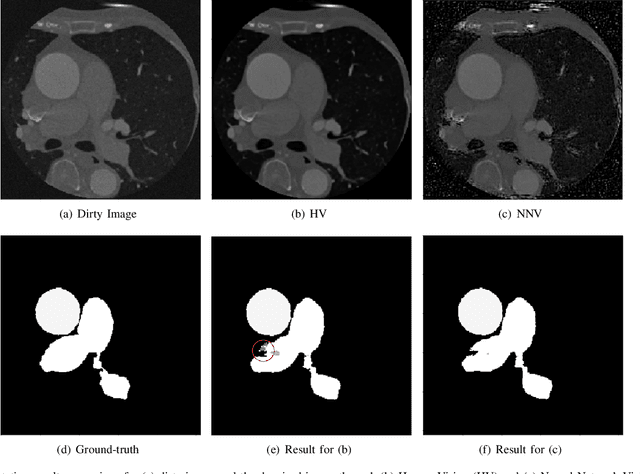
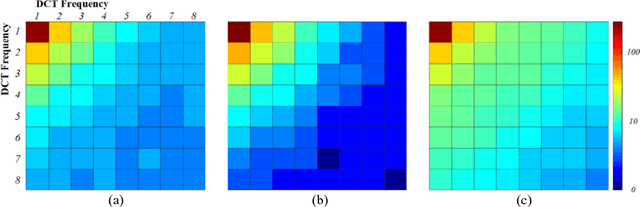
Abstract:Deep learning had already demonstrated its power in medical images, including denoising, classification, segmentation, etc. All these applications are proposed to automatically analyze medical images beforehand, which brings more information to radiologists during clinical assessment for accuracy improvement. Recently, many medical denoising methods had shown their significant artifact reduction result and noise removal both quantitatively and qualitatively. However, those existing methods are developed around human-vision, i.e., they are designed to minimize the noise effect that can be perceived by human eyes. In this paper, we introduce an application-guided denoising framework, which focuses on denoising for the following neural networks. In our experiments, we apply the proposed framework to different datasets, models, and use cases. Experimental results show that our proposed framework can achieve a better result than human-vision denoising network.
ICA-UNet: ICA Inspired Statistical UNet for Real-time 3D Cardiac Cine MRI Segmentation
Jul 18, 2020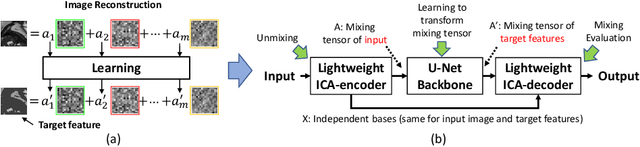
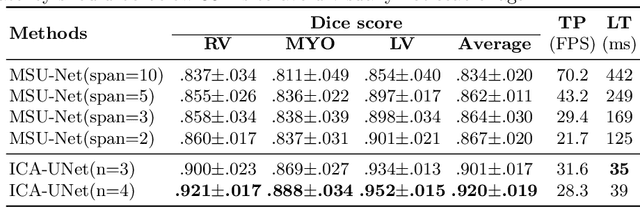
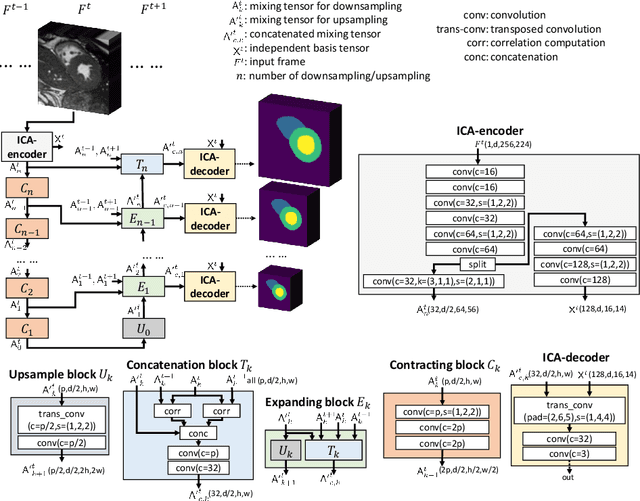
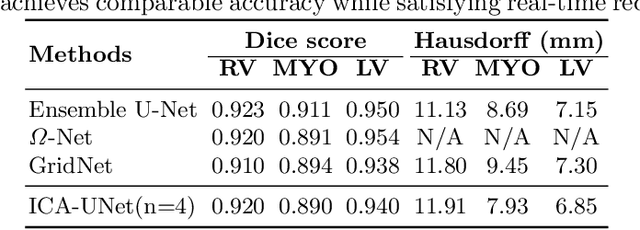
Abstract:Real-time cine magnetic resonance imaging (MRI) plays an increasingly important role in various cardiac interventions. In order to enable fast and accurate visual assistance, the temporal frames need to be segmented on-the-fly. However, state-of-the-art MRI segmentation methods are used either offline because of their high computation complexity, or in real-time but with significant accuracy loss and latency increase (causing visually noticeable lag). As such, they can hardly be adopted to assist visual guidance. In this work, inspired by a new interpretation of Independent Component Analysis (ICA) for learning, we propose a novel ICA-UNet for real-time 3D cardiac cine MRI segmentation. Experiments using the MICCAI ACDC 2017 dataset show that, compared with the state-of-the-arts, ICA-UNet not only achieves higher Dice scores, but also meets the real-time requirements for both throughput and latency (up to 12.6X reduction), enabling real-time guidance for cardiac interventions without visual lag.
Multi-Cycle-Consistent Adversarial Networks for CT Image Denoising
Feb 27, 2020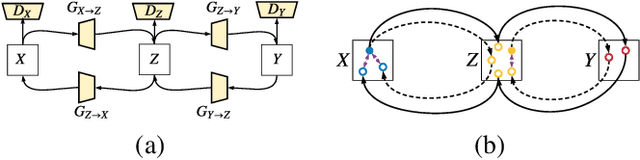
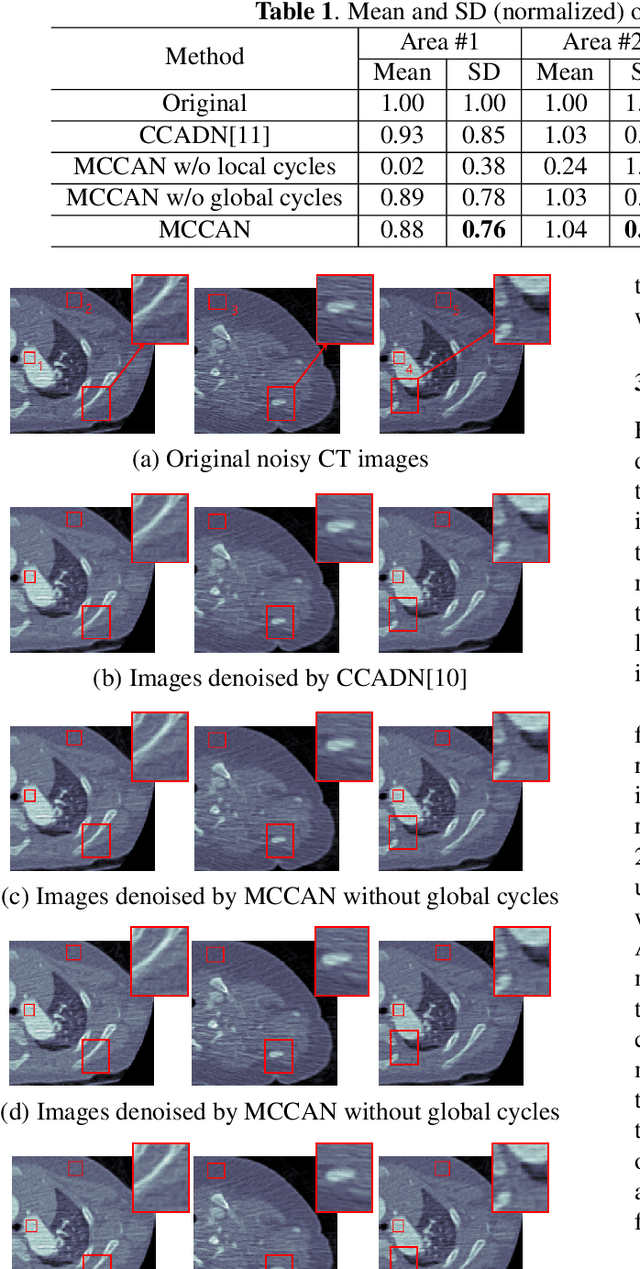
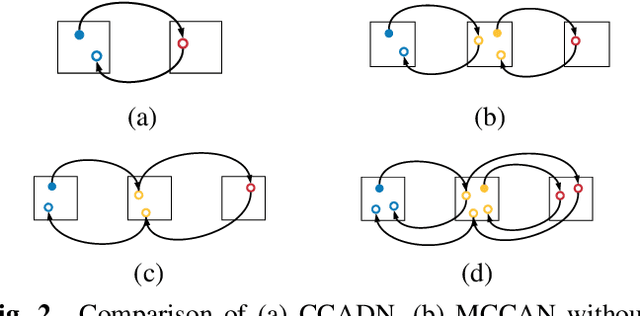
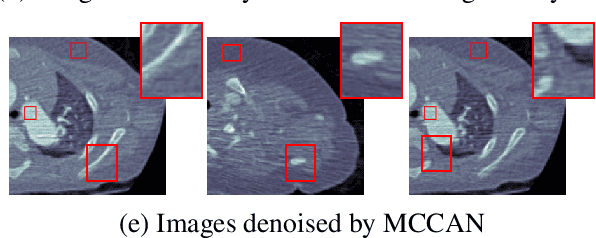
Abstract:CT image denoising can be treated as an image-to-image translation task where the goal is to learn the transform between a source domain $X$ (noisy images) and a target domain $Y$ (clean images). Recently, cycle-consistent adversarial denoising network (CCADN) has achieved state-of-the-art results by enforcing cycle-consistent loss without the need of paired training data. Our detailed analysis of CCADN raises a number of interesting questions. For example, if the noise is large leading to significant difference between domain $X$ and domain $Y$, can we bridge $X$ and $Y$ with an intermediate domain $Z$ such that both the denoising process between $X$ and $Z$ and that between $Z$ and $Y$ are easier to learn? As such intermediate domains lead to multiple cycles, how do we best enforce cycle-consistency? Driven by these questions, we propose a multi-cycle-consistent adversarial network (MCCAN) that builds intermediate domains and enforces both local and global cycle-consistency. The global cycle-consistency couples all generators together to model the whole denoising process, while the local cycle-consistency imposes effective supervision on the process between adjacent domains. Experiments show that both local and global cycle-consistency are important for the success of MCCAN, which outperforms the state-of-the-art.
Accurate Congenital Heart Disease Model Generation for 3D Printing
Jul 12, 2019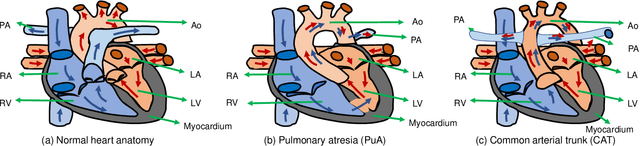
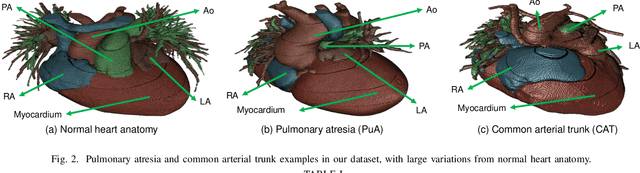
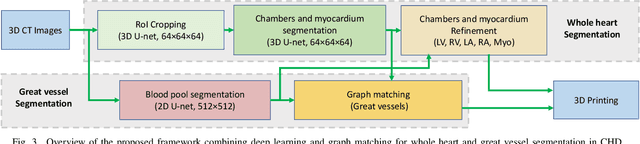
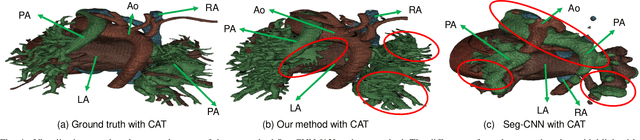
Abstract:3D printing has been widely adopted for clinical decision making and interventional planning of Congenital heart disease (CHD), while whole heart and great vessel segmentation is the most significant but time-consuming step in the model generation for 3D printing. While various automatic whole heart and great vessel segmentation frameworks have been developed in the literature, they are ineffective when applied to medical images in CHD, which have significant variations in heart structure and great vessel connections. To address the challenge, we leverage the power of deep learning in processing regular structures and that of graph algorithms in dealing with large variations and propose a framework that combines both for whole heart and great vessel segmentation in CHD. Particularly, we first use deep learning to segment the four chambers and myocardium followed by the blood pool, where variations are usually small. We then extract the connection information and apply graph matching to determine the categories of all the vessels. Experimental results using 683D CT images covering 14 types of CHD show that our method can increase Dice score by 11.9% on average compared with the state-of-the-art whole heart and great vessel segmentation method in normal anatomy. The segmentation results are also printed out using 3D printers for validation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge