Moritz Rempe
Efficient Complex-Valued Vision Transformers for MRI Classification Directly from k-Space
Jan 26, 2026Abstract:Deep learning applications in Magnetic Resonance Imaging (MRI) predominantly operate on reconstructed magnitude images, a process that discards phase information and requires computationally expensive transforms. Standard neural network architectures rely on local operations (convolutions or grid-patches) that are ill-suited for the global, non-local nature of raw frequency-domain (k-Space) data. In this work, we propose a novel complex-valued Vision Transformer (kViT) designed to perform classification directly on k-Space data. To bridge the geometric disconnect between current architectures and MRI physics, we introduce a radial k-Space patching strategy that respects the spectral energy distribution of the frequency-domain. Extensive experiments on the fastMRI and in-house datasets demonstrate that our approach achieves classification performance competitive with state-of-the-art image-domain baselines (ResNet, EfficientNet, ViT). Crucially, kViT exhibits superior robustness to high acceleration factors and offers a paradigm shift in computational efficiency, reducing VRAM consumption during training by up to 68$\times$ compared to standard methods. This establishes a pathway for resource-efficient, direct-from-scanner AI analysis.
PhaseGen: A Diffusion-Based Approach for Complex-Valued MRI Data Generation
Apr 10, 2025Abstract:Magnetic resonance imaging (MRI) raw data, or k-Space data, is complex-valued, containing both magnitude and phase information. However, clinical and existing Artificial Intelligence (AI)-based methods focus only on magnitude images, discarding the phase data despite its potential for downstream tasks, such as tumor segmentation and classification. In this work, we introduce $\textit{PhaseGen}$, a novel complex-valued diffusion model for generating synthetic MRI raw data conditioned on magnitude images, commonly used in clinical practice. This enables the creation of artificial complex-valued raw data, allowing pretraining for models that require k-Space information. We evaluate PhaseGen on two tasks: skull-stripping directly in k-Space and MRI reconstruction using the publicly available FastMRI dataset. Our results show that training with synthetic phase data significantly improves generalization for skull-stripping on real-world data, with an increased segmentation accuracy from $41.1\%$ to $80.1\%$, and enhances MRI reconstruction when combined with limited real-world data. This work presents a step forward in utilizing generative AI to bridge the gap between magnitude-based datasets and the complex-valued nature of MRI raw data. This approach allows researchers to leverage the vast amount of avaliable image domain data in combination with the information-rich k-Space data for more accurate and efficient diagnostic tasks. We make our code publicly $\href{https://github.com/TIO-IKIM/PhaseGen}{\text{available here}}$.
Cracking the PUMA Challenge in 24 Hours with CellViT++ and nnU-Net
Mar 15, 2025
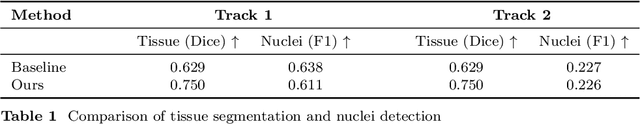
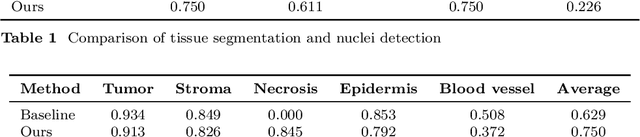
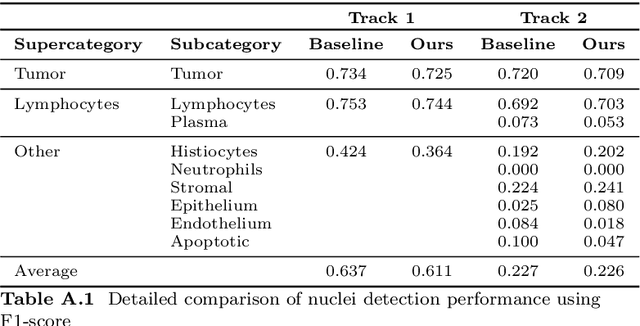
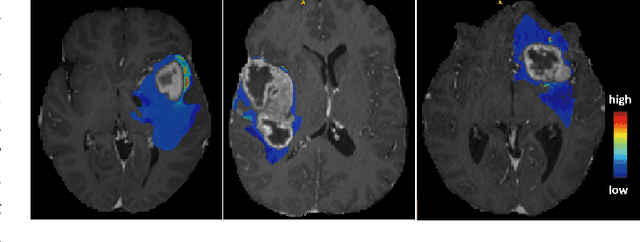
Abstract:Automatic tissue segmentation and nuclei detection is an important task in pathology, aiding in biomarker extraction and discovery. The panoptic segmentation of nuclei and tissue in advanced melanoma (PUMA) challenge aims to improve tissue segmentation and nuclei detection in melanoma histopathology. Unlike many challenge submissions focusing on extensive model tuning, our approach emphasizes delivering a deployable solution within a 24-hour development timeframe, using out-of-the-box frameworks. The pipeline combines two models, namely CellViT++ for nuclei detection and nnU-Net for tissue segmentation. Our results demonstrate a significant improvement in tissue segmentation, achieving a Dice score of 0.750, surpassing the baseline score of 0.629. For nuclei detection, we obtained results comparable to the baseline in both challenge tracks. The code is publicly available at https://github.com/TIO-IKIM/PUMA.
CellViT++: Energy-Efficient and Adaptive Cell Segmentation and Classification Using Foundation Models
Jan 09, 2025



Abstract:Digital Pathology is a cornerstone in the diagnosis and treatment of diseases. A key task in this field is the identification and segmentation of cells in hematoxylin and eosin-stained images. Existing methods for cell segmentation often require extensive annotated datasets for training and are limited to a predefined cell classification scheme. To overcome these limitations, we propose $\text{CellViT}^{{\scriptscriptstyle ++}}$, a framework for generalized cell segmentation in digital pathology. $\text{CellViT}^{{\scriptscriptstyle ++}}$ utilizes Vision Transformers with foundation models as encoders to compute deep cell features and segmentation masks simultaneously. To adapt to unseen cell types, we rely on a computationally efficient approach. It requires minimal data for training and leads to a drastically reduced carbon footprint. We demonstrate excellent performance on seven different datasets, covering a broad spectrum of cell types, organs, and clinical settings. The framework achieves remarkable zero-shot segmentation and data-efficient cell-type classification. Furthermore, we show that $\text{CellViT}^{{\scriptscriptstyle ++}}$ can leverage immunofluorescence stainings to generate training datasets without the need for pathologist annotations. The automated dataset generation approach surpasses the performance of networks trained on manually labeled data, demonstrating its effectiveness in creating high-quality training datasets without expert annotations. To advance digital pathology, $\text{CellViT}^{{\scriptscriptstyle ++}}$ is available as an open-source framework featuring a user-friendly, web-based interface for visualization and annotation. The code is available under https://github.com/TIO-IKIM/CellViT-plus-plus.
De-Identification of Medical Imaging Data: A Comprehensive Tool for Ensuring Patient Privacy
Oct 16, 2024



Abstract:Medical data employed in research frequently comprises sensitive patient health information (PHI), which is subject to rigorous legal frameworks such as the General Data Protection Regulation (GDPR) or the Health Insurance Portability and Accountability Act (HIPAA). Consequently, these types of data must be pseudonymized prior to utilisation, which presents a significant challenge for many researchers. Given the vast array of medical data, it is necessary to employ a variety of de-identification techniques. To facilitate the anonymization process for medical imaging data, we have developed an open-source tool that can be used to de-identify DICOM magnetic resonance images, computer tomography images, whole slide images and magnetic resonance twix raw data. Furthermore, the implementation of a neural network enables the removal of text within the images. The proposed tool automates an elaborate anonymization pipeline for multiple types of inputs, reducing the need for additional tools used for de-identification of imaging data. We make our code publicly available at https://github.com/code-lukas/medical_image_deidentification.
MedShapeNet -- A Large-Scale Dataset of 3D Medical Shapes for Computer Vision
Sep 12, 2023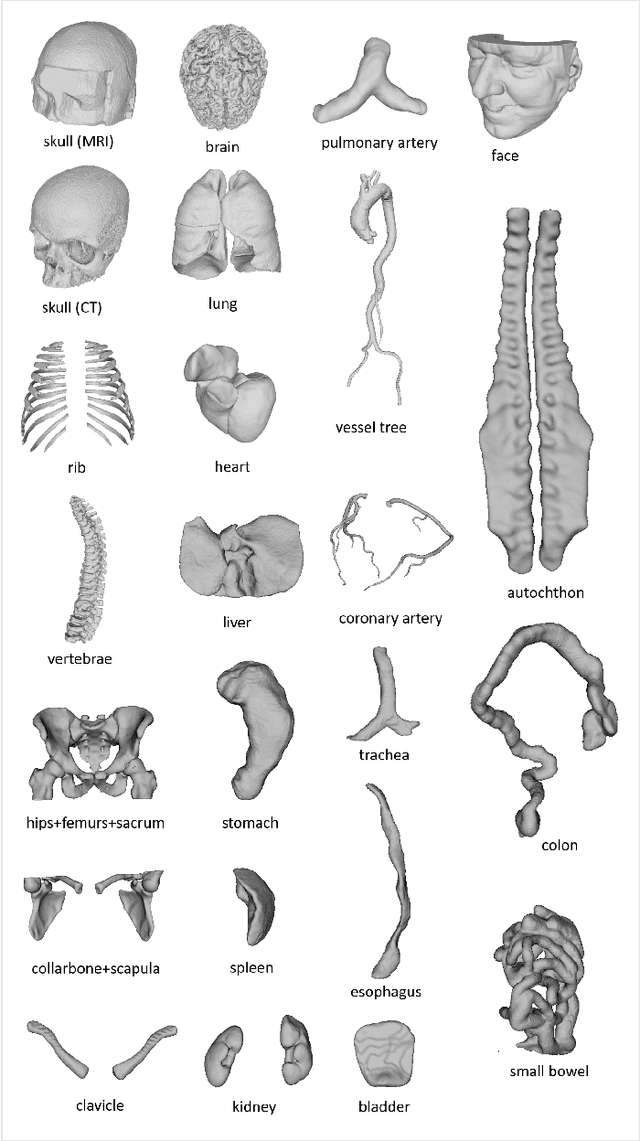

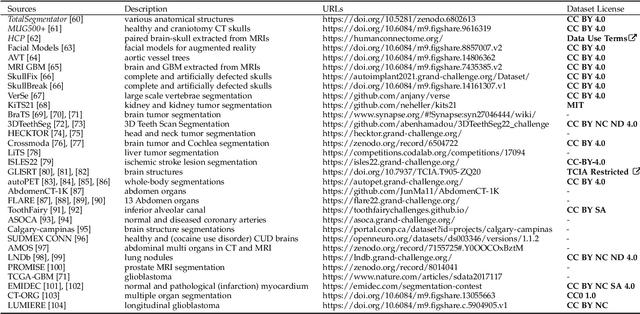
Abstract:We present MedShapeNet, a large collection of anatomical shapes (e.g., bones, organs, vessels) and 3D surgical instrument models. Prior to the deep learning era, the broad application of statistical shape models (SSMs) in medical image analysis is evidence that shapes have been commonly used to describe medical data. Nowadays, however, state-of-the-art (SOTA) deep learning algorithms in medical imaging are predominantly voxel-based. In computer vision, on the contrary, shapes (including, voxel occupancy grids, meshes, point clouds and implicit surface models) are preferred data representations in 3D, as seen from the numerous shape-related publications in premier vision conferences, such as the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), as well as the increasing popularity of ShapeNet (about 51,300 models) and Princeton ModelNet (127,915 models) in computer vision research. MedShapeNet is created as an alternative to these commonly used shape benchmarks to facilitate the translation of data-driven vision algorithms to medical applications, and it extends the opportunities to adapt SOTA vision algorithms to solve critical medical problems. Besides, the majority of the medical shapes in MedShapeNet are modeled directly on the imaging data of real patients, and therefore it complements well existing shape benchmarks comprising of computer-aided design (CAD) models. MedShapeNet currently includes more than 100,000 medical shapes, and provides annotations in the form of paired data. It is therefore also a freely available repository of 3D models for extended reality (virtual reality - VR, augmented reality - AR, mixed reality - MR) and medical 3D printing. This white paper describes in detail the motivations behind MedShapeNet, the shape acquisition procedures, the use cases, as well as the usage of the online shape search portal: https://medshapenet.ikim.nrw/
CellViT: Vision Transformers for Precise Cell Segmentation and Classification
Jun 27, 2023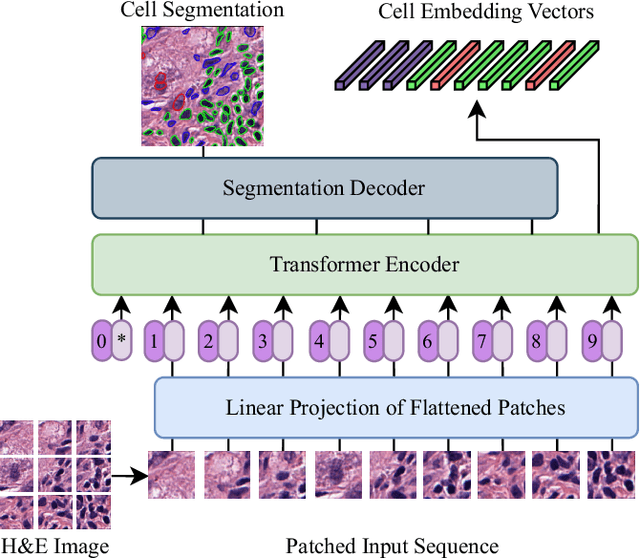
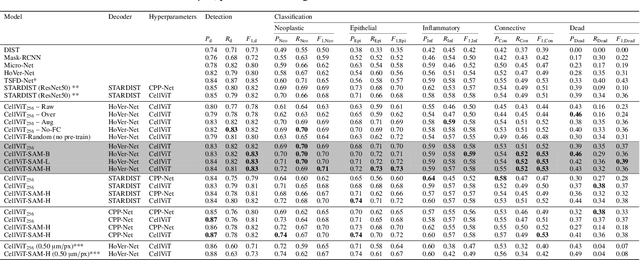
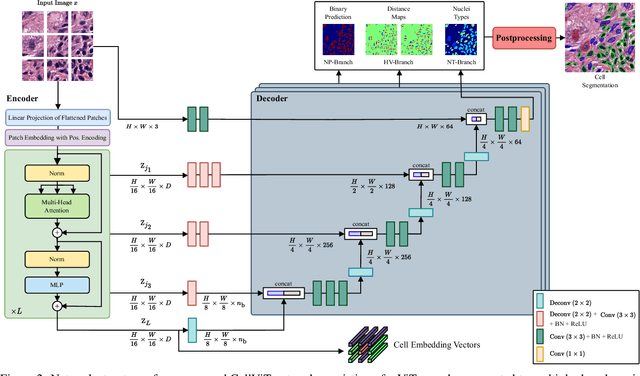

Abstract:Nuclei detection and segmentation in hematoxylin and eosin-stained (H&E) tissue images are important clinical tasks and crucial for a wide range of applications. However, it is a challenging task due to nuclei variances in staining and size, overlapping boundaries, and nuclei clustering. While convolutional neural networks have been extensively used for this task, we explore the potential of Transformer-based networks in this domain. Therefore, we introduce a new method for automated instance segmentation of cell nuclei in digitized tissue samples using a deep learning architecture based on Vision Transformer called CellViT. CellViT is trained and evaluated on the PanNuke dataset, which is one of the most challenging nuclei instance segmentation datasets, consisting of nearly 200,000 annotated Nuclei into 5 clinically important classes in 19 tissue types. We demonstrate the superiority of large-scale in-domain and out-of-domain pre-trained Vision Transformers by leveraging the recently published Segment Anything Model and a ViT-encoder pre-trained on 104 million histological image patches - achieving state-of-the-art nuclei detection and instance segmentation performance on the PanNuke dataset with a mean panoptic quality of 0.51 and an F1-detection score of 0.83. The code is publicly available at https://github.com/TIO-IKIM/CellViT
k-strip: A novel segmentation algorithm in k-space for the application of skull stripping
May 19, 2022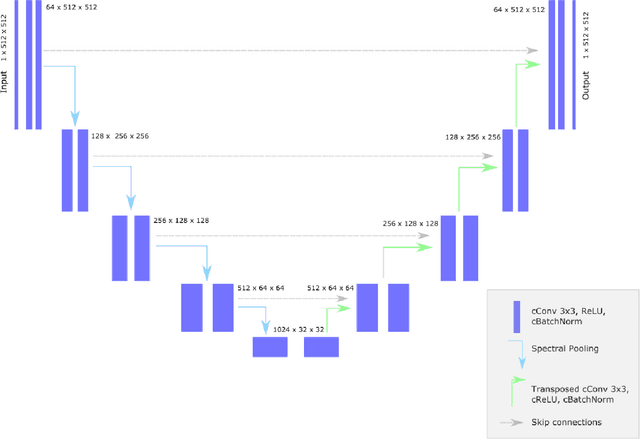
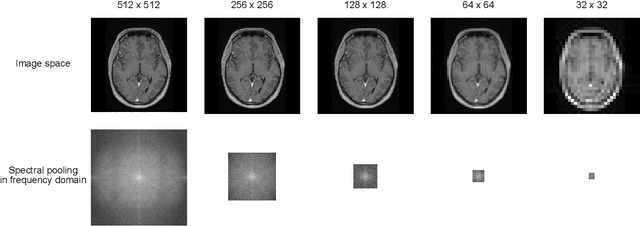
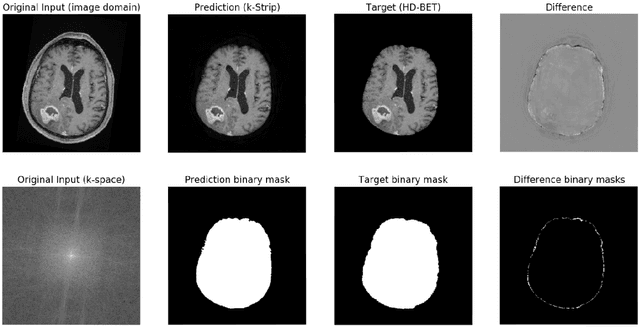
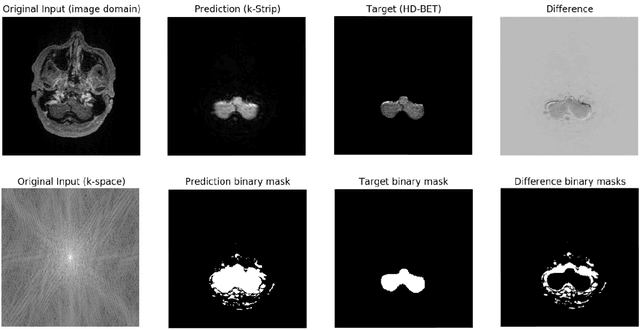
Abstract:Objectives: Present a novel deep learning-based skull stripping algorithm for magnetic resonance imaging (MRI) that works directly in the information rich k-space. Materials and Methods: Using two datasets from different institutions with a total of 36,900 MRI slices, we trained a deep learning-based model to work directly with the complex raw k-space data. Skull stripping performed by HD-BET (Brain Extraction Tool) in the image domain were used as the ground truth. Results: Both datasets were very similar to the ground truth (DICE scores of 92\%-98\% and Hausdorff distances of under 5.5 mm). Results on slices above the eye-region reach DICE scores of up to 99\%, while the accuracy drops in regions around the eyes and below, with partially blurred output. The output of k-strip often smoothed edges at the demarcation to the skull. Binary masks are created with an appropriate threshold. Conclusion: With this proof-of-concept study, we were able to show the feasibility of working in the k-space frequency domain, preserving phase information, with consistent results. Future research should be dedicated to discovering additional ways the k-space can be used for innovative image analysis and further workflows.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge