Mohamed Elshaikh
Hybrid Student-Teacher Large Language Model Refinement for Cancer Toxicity Symptom Extraction
Aug 08, 2024


Abstract:Large Language Models (LLMs) offer significant potential for clinical symptom extraction, but their deployment in healthcare settings is constrained by privacy concerns, computational limitations, and operational costs. This study investigates the optimization of compact LLMs for cancer toxicity symptom extraction using a novel iterative refinement approach. We employ a student-teacher architecture, utilizing Zephyr-7b-beta and Phi3-mini-128 as student models and GPT-4o as the teacher, to dynamically select between prompt refinement, Retrieval-Augmented Generation (RAG), and fine-tuning strategies. Our experiments on 294 clinical notes covering 12 post-radiotherapy toxicity symptoms demonstrate the effectiveness of this approach. The RAG method proved most efficient, improving average accuracy scores from 0.32 to 0.73 for Zephyr-7b-beta and from 0.40 to 0.87 for Phi3-mini-128 during refinement. In the test set, both models showed an approximate 0.20 increase in accuracy across symptoms. Notably, this improvement was achieved at a cost 45 times lower than GPT-4o for Zephyr and 79 times lower for Phi-3. These results highlight the potential of iterative refinement techniques in enhancing the capabilities of compact LLMs for clinical applications, offering a balance between performance, cost-effectiveness, and privacy preservation in healthcare settings.
Iterative Prompt Refinement for Radiation Oncology Symptom Extraction Using Teacher-Student Large Language Models
Feb 06, 2024Abstract:This study introduces a novel teacher-student architecture utilizing Large Language Models (LLMs) to improve prostate cancer radiotherapy symptom extraction from clinical notes. Mixtral, the student model, initially extracts symptoms, followed by GPT-4, the teacher model, which refines prompts based on Mixtral's performance. This iterative process involved 294 single symptom clinical notes across 12 symptoms, with up to 16 rounds of refinement per epoch. Results showed significant improvements in extracting symptoms from both single and multi-symptom notes. For 59 single symptom notes, accuracy increased from 0.51 to 0.71, precision from 0.52 to 0.82, recall from 0.52 to 0.72, and F1 score from 0.49 to 0.73. In 375 multi-symptom notes, accuracy rose from 0.24 to 0.43, precision from 0.6 to 0.76, recall from 0.24 to 0.43, and F1 score from 0.20 to 0.44. These results demonstrate the effectiveness of advanced prompt engineering in LLMs for radiation oncology use.
An Introduction to Natural Language Processing Techniques and Framework for Clinical Implementation in Radiation Oncology
Nov 08, 2023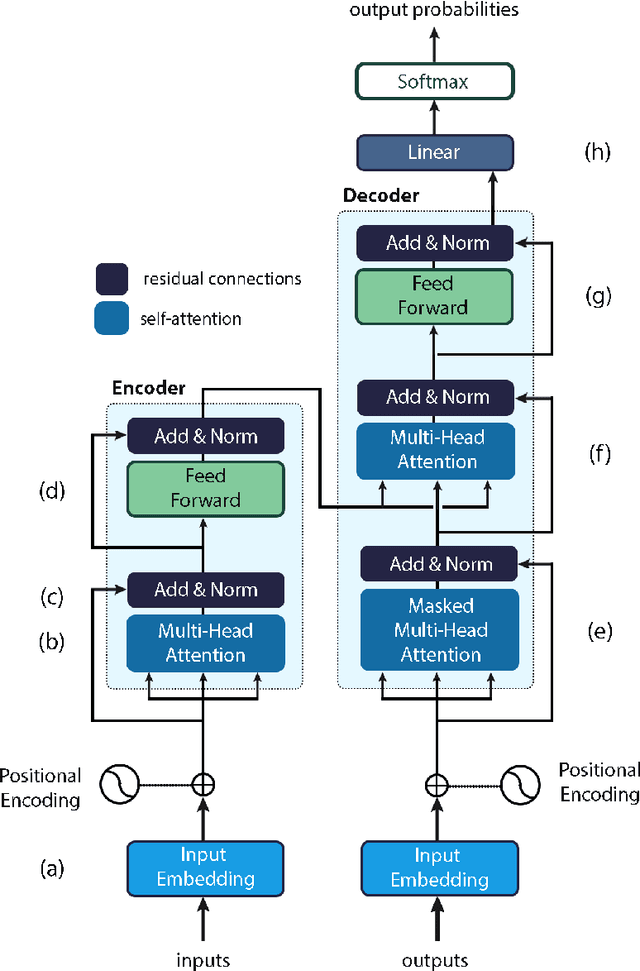

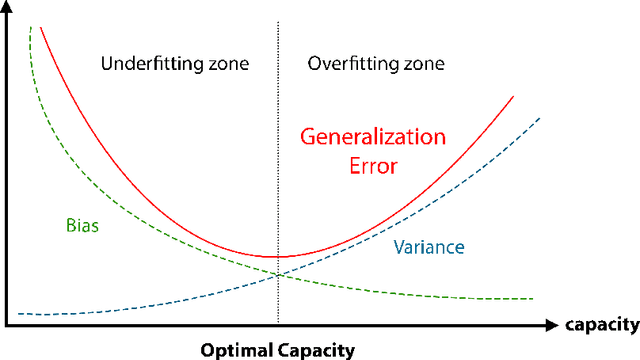
Abstract:Natural Language Processing (NLP) is a key technique for developing Medical Artificial Intelligence (AI) systems that leverage Electronic Health Record (EHR) data to build diagnostic and prognostic models. NLP enables the conversion of unstructured clinical text into structured data that can be fed into AI algorithms. The emergence of the transformer architecture and large language models (LLMs) has led to remarkable advances in NLP for various healthcare tasks, such as entity recognition, relation extraction, sentence similarity, text summarization, and question answering. In this article, we review the major technical innovations that underpin modern NLP models and present state-of-the-art NLP applications that employ LLMs in radiation oncology research. However, these LLMs are prone to many errors such as hallucinations, biases, and ethical violations, which necessitate rigorous evaluation and validation before clinical deployment. As such, we propose a comprehensive framework for assessing the NLP models based on their purpose and clinical fit, technical performance, bias and trust, legal and ethical implications, and quality assurance, prior to implementation in clinical radiation oncology. Our article aims to provide guidance and insights for researchers and clinicians who are interested in developing and using NLP models in clinical radiation oncology.
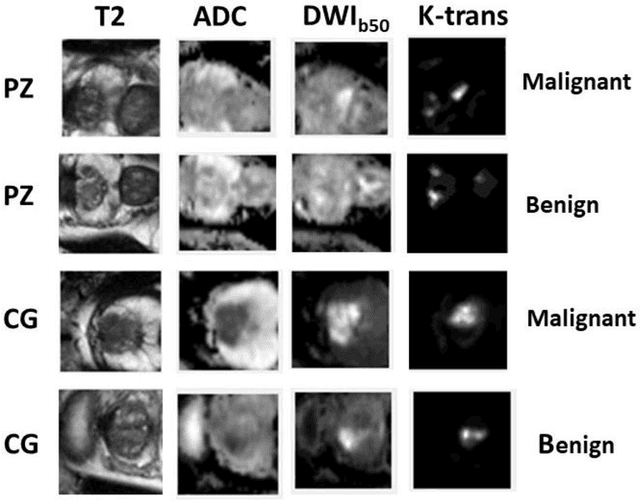
Accurate Prostate Cancer Detection and Segmentation on Biparametric MRI using Non-local Mask R-CNN with Histopathological Ground Truth
Oct 28, 2020



Abstract:Purpose: We aimed to develop deep machine learning (DL) models to improve the detection and segmentation of intraprostatic lesions (IL) on bp-MRI by using whole amount prostatectomy specimen-based delineations. We also aimed to investigate whether transfer learning and self-training would improve results with small amount labelled data. Methods: 158 patients had suspicious lesions delineated on MRI based on bp-MRI, 64 patients had ILs delineated on MRI based on whole mount prostatectomy specimen sections, 40 patients were unlabelled. A non-local Mask R-CNN was proposed to improve the segmentation accuracy. Transfer learning was investigated by fine-tuning a model trained using MRI-based delineations with prostatectomy-based delineations. Two label selection strategies were investigated in self-training. The performance of models was evaluated by 3D detection rate, dice similarity coefficient (DSC), 95 percentile Hausdrauff (95 HD, mm) and true positive ratio (TPR). Results: With prostatectomy-based delineations, the non-local Mask R-CNN with fine-tuning and self-training significantly improved all evaluation metrics. For the model with the highest detection rate and DSC, 80.5% (33/41) of lesions in all Gleason Grade Groups (GGG) were detected with DSC of 0.548[0.165], 95 HD of 5.72[3.17] and TPR of 0.613[0.193]. Among them, 94.7% (18/19) of lesions with GGG > 2 were detected with DSC of 0.604[0.135], 95 HD of 6.26[3.44] and TPR of 0.580[0.190]. Conclusion: DL models can achieve high prostate cancer detection and segmentation accuracy on bp-MRI based on annotations from histologic images. To further improve the performance, more data with annotations of both MRI and whole amount prostatectomy specimens are required.
A Deep Dive into Understanding Tumor Foci Classification using Multiparametric MRI Based on Convolutional Neural Network
Apr 04, 2019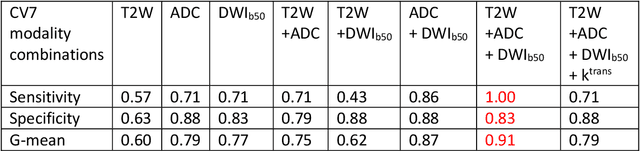
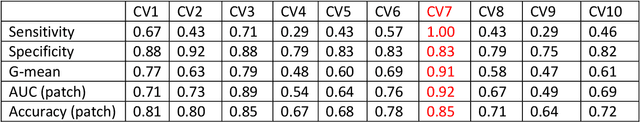
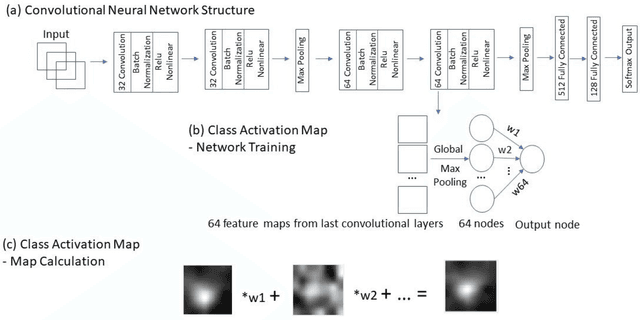
Abstract:Data scarcity has refrained deep learning models from making greater progress in prostate images analysis using multiparametric MRI. In this paper, an efficient convolutional neural network (CNN) was developed to classify lesion malignancy for prostate cancer patients, based on which model interpretation was systematically analyzed to bridge the gap between natural images and MR images, which is the first one of its kind in the literature. The problem of small sample size was addressed and successfully tackled by feeding the intermediate features into a traditional classification algorithm known as weighted extreme learning machine, with imbalanced distribution among output categories taken into consideration. Model trained on public data set was able to generalize to data from an independent institution to make accurate prediction. The generated saliency map was found to overlay well with the lesion and could benefit clinicians for diagnosing purpose.
Segmentation of the Prostatic Gland and the Intraprostatic Lesions on Multiparametic MRI Using Mask-RCNN
Apr 04, 2019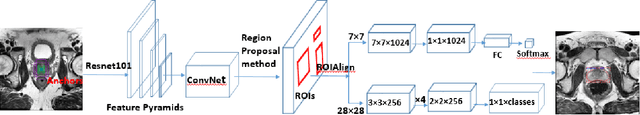
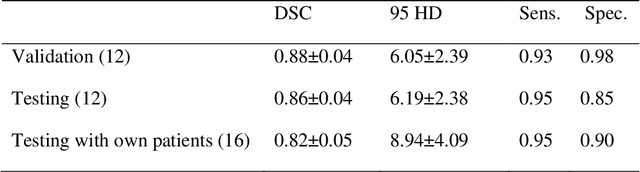
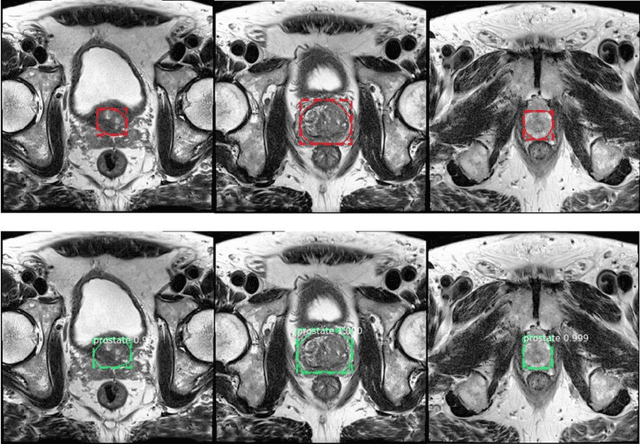
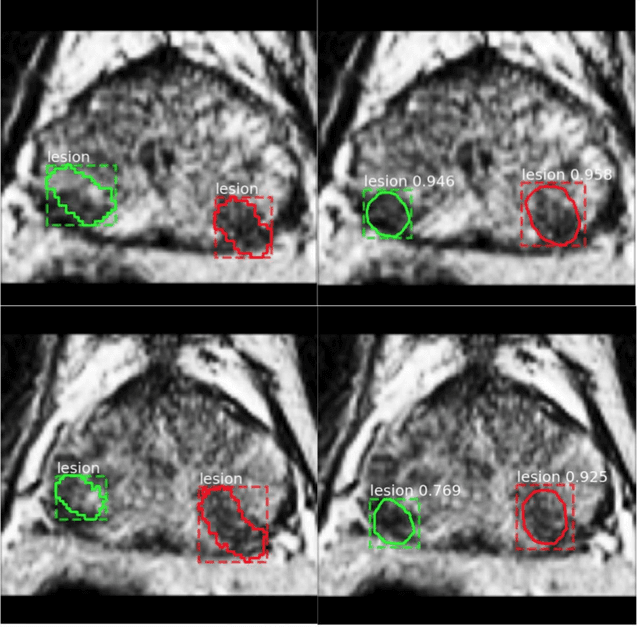
Abstract:Prostate cancer (PCa) is the most common cancer in men in the United States. Multiparametic magnetic resonance imaging (mp-MRI) has been explored by many researchers to targeted prostate biopsies and radiation therapy. However, assessment on mp-MRI can be subjective, development of computer-aided diagnosis systems to automatically delineate the prostate gland and the intraprostratic lesions (ILs) becomes important to facilitate with radiologists in clinical practice. In this paper, we first study the implementation of the Mask-RCNN model to segment the prostate and ILs. We trained and evaluated models on 120 patients from two different cohorts of patients. We also used 2D U-Net and 3D U-Net as benchmarks to segment the prostate and compared the model's performance. The contour variability of ILs using the algorithm was also benchmarked against the interobserver variability between two different radiation oncologists on 19 patients. Our results indicate that the Mask-RCNN model is able to reach state-of-art performance in the prostate segmentation and outperforms several competitive baselines in ILs segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge