Zhenzhen Dai
Accurate Prostate Cancer Detection and Segmentation on Biparametric MRI using Non-local Mask R-CNN with Histopathological Ground Truth
Oct 28, 2020



Abstract:Purpose: We aimed to develop deep machine learning (DL) models to improve the detection and segmentation of intraprostatic lesions (IL) on bp-MRI by using whole amount prostatectomy specimen-based delineations. We also aimed to investigate whether transfer learning and self-training would improve results with small amount labelled data. Methods: 158 patients had suspicious lesions delineated on MRI based on bp-MRI, 64 patients had ILs delineated on MRI based on whole mount prostatectomy specimen sections, 40 patients were unlabelled. A non-local Mask R-CNN was proposed to improve the segmentation accuracy. Transfer learning was investigated by fine-tuning a model trained using MRI-based delineations with prostatectomy-based delineations. Two label selection strategies were investigated in self-training. The performance of models was evaluated by 3D detection rate, dice similarity coefficient (DSC), 95 percentile Hausdrauff (95 HD, mm) and true positive ratio (TPR). Results: With prostatectomy-based delineations, the non-local Mask R-CNN with fine-tuning and self-training significantly improved all evaluation metrics. For the model with the highest detection rate and DSC, 80.5% (33/41) of lesions in all Gleason Grade Groups (GGG) were detected with DSC of 0.548[0.165], 95 HD of 5.72[3.17] and TPR of 0.613[0.193]. Among them, 94.7% (18/19) of lesions with GGG > 2 were detected with DSC of 0.604[0.135], 95 HD of 6.26[3.44] and TPR of 0.580[0.190]. Conclusion: DL models can achieve high prostate cancer detection and segmentation accuracy on bp-MRI based on annotations from histologic images. To further improve the performance, more data with annotations of both MRI and whole amount prostatectomy specimens are required.
Improvement of Multiparametric MR Image Segmentation by Augmenting the Data with Generative Adversarial Networks for Glioma Patients
Oct 01, 2019



Abstract:Every year thousands of patients are diagnosed with a glioma, a type of malignant brain tumor. Physicians use MR images as a key tool in the diagnosis and treatment of these patients. Neural networks show great potential to aid physicians in the medical image analysis. This study investigates the use of varying amounts of synthetic brain T1-weighted (T1), post-contrast T1-weighted (T1Gd), T2-weighted (T2), and T2 Fluid Attenuated Inversion Recovery (FLAIR) MR images created by a generative adversarial network to overcome the lack of annotated medical image data in training separate 2D U-Nets to segment enhancing tumor, peritumoral edema, and necrosis (non-enhancing tumor core) regions on gliomas. These synthetic MR images were assessed quantitively (SSIM=0.79) and qualitatively by a physician who found that the synthetic images seem stronger for delineation of structural boundaries but struggle more when gradient is significant, (e.g. edema signal in T2 modalities). Multiple 2D U-Nets were trained with original BraTS data and differing subsets of a quarter, half, three-quarters, and all synthetic MR images. There was not an obvious correlation between the improvement of values of the metrics in separate validation dataset for each structure and amount of synthetic data added, there is a strong correlation between the amount of synthetic data added and the number of best overall validation metrics. In summary, this study showed ability to generate high quality synthetic Flair, T2, T1, and T1CE MR images using the GAN. Using the synthetic MR images showed encouraging results to improve the U-Net segmentation performance which has the potential to address the scarcity of readily available medical images.
Segmentation of the Prostatic Gland and the Intraprostatic Lesions on Multiparametic MRI Using Mask-RCNN
Apr 04, 2019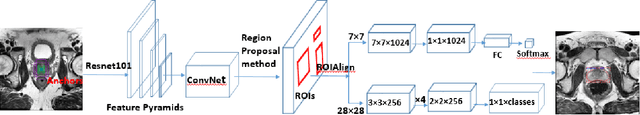
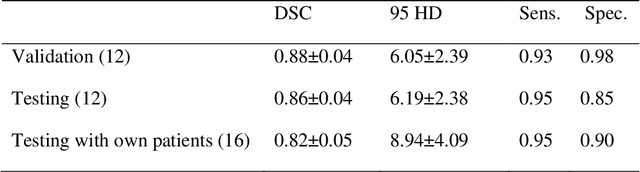
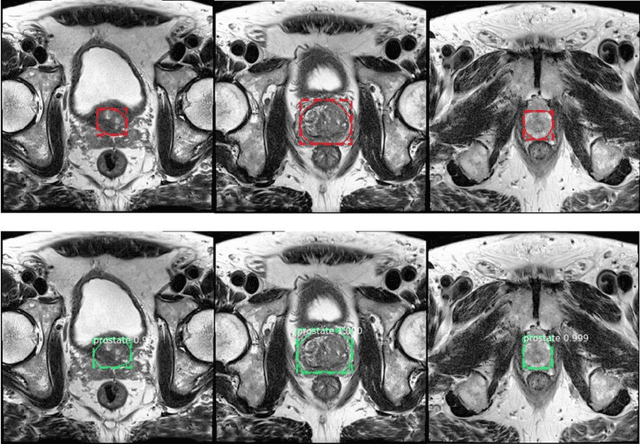
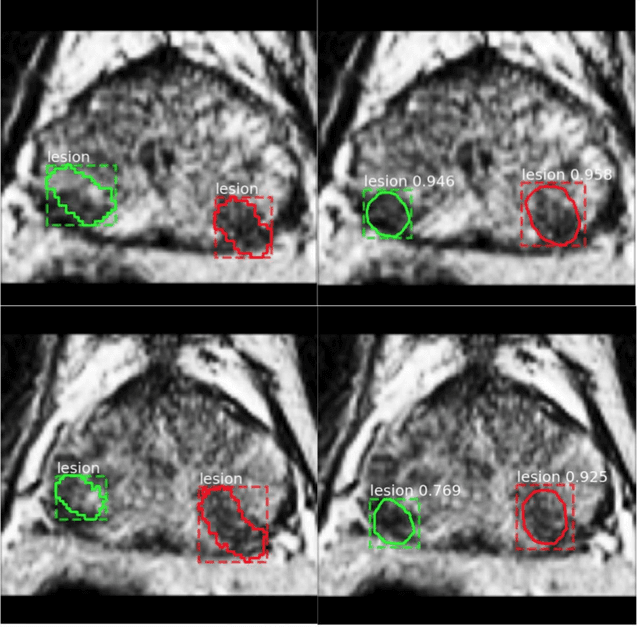
Abstract:Prostate cancer (PCa) is the most common cancer in men in the United States. Multiparametic magnetic resonance imaging (mp-MRI) has been explored by many researchers to targeted prostate biopsies and radiation therapy. However, assessment on mp-MRI can be subjective, development of computer-aided diagnosis systems to automatically delineate the prostate gland and the intraprostratic lesions (ILs) becomes important to facilitate with radiologists in clinical practice. In this paper, we first study the implementation of the Mask-RCNN model to segment the prostate and ILs. We trained and evaluated models on 120 patients from two different cohorts of patients. We also used 2D U-Net and 3D U-Net as benchmarks to segment the prostate and compared the model's performance. The contour variability of ILs using the algorithm was also benchmarked against the interobserver variability between two different radiation oncologists on 19 patients. Our results indicate that the Mask-RCNN model is able to reach state-of-art performance in the prostate segmentation and outperforms several competitive baselines in ILs segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge