Hassan Bagher-Ebadian
Automated stereotactic radiosurgery planning using a human-in-the-loop reasoning large language model agent
Dec 23, 2025



Abstract:Stereotactic radiosurgery (SRS) demands precise dose shaping around critical structures, yet black-box AI systems have limited clinical adoption due to opacity concerns. We tested whether chain-of-thought reasoning improves agentic planning in a retrospective cohort of 41 patients with brain metastases treated with 18 Gy single-fraction SRS. We developed SAGE (Secure Agent for Generative Dose Expertise), an LLM-based planning agent for automated SRS treatment planning. Two variants generated plans for each case: one using a non-reasoning model, one using a reasoning model. The reasoning variant showed comparable plan dosimetry relative to human planners on primary endpoints (PTV coverage, maximum dose, conformity index, gradient index; all p > 0.21) while reducing cochlear dose below human baselines (p = 0.022). When prompted to improve conformity, the reasoning model demonstrated systematic planning behaviors including prospective constraint verification (457 instances) and trade-off deliberation (609 instances), while the standard model exhibited none of these deliberative processes (0 and 7 instances, respectively). Content analysis revealed that constraint verification and causal explanation concentrated in the reasoning agent. The optimization traces serve as auditable logs, offering a path toward transparent automated planning.
GLoG-CSUnet: Enhancing Vision Transformers with Adaptable Radiomic Features for Medical Image Segmentation
Jan 08, 2025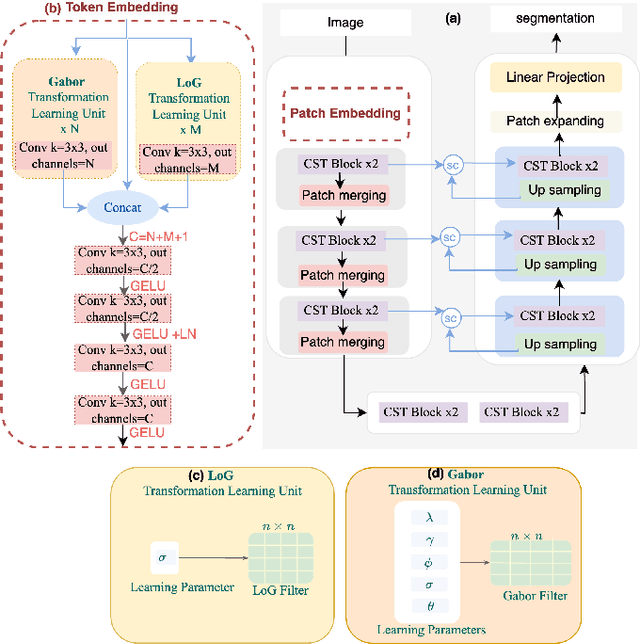
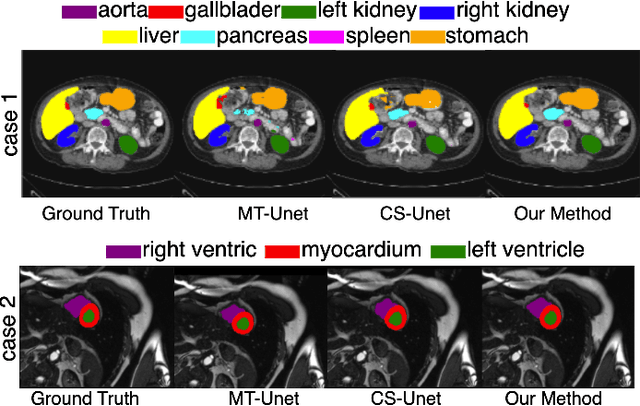
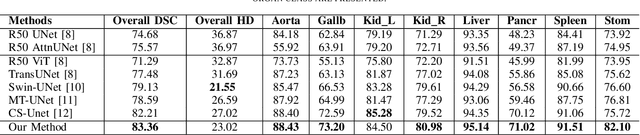
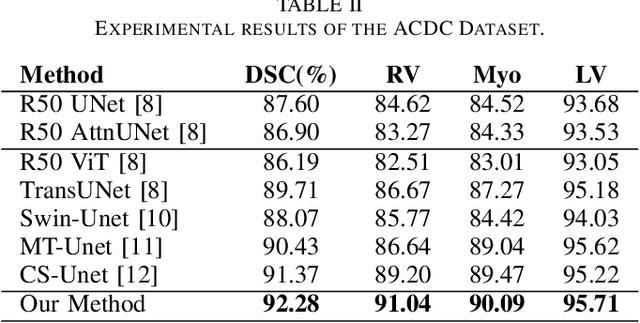
Abstract:Vision Transformers (ViTs) have shown promise in medical image semantic segmentation (MISS) by capturing long-range correlations. However, ViTs often struggle to model local spatial information effectively, which is essential for accurately segmenting fine anatomical details, particularly when applied to small datasets without extensive pre-training. We introduce Gabor and Laplacian of Gaussian Convolutional Swin Network (GLoG-CSUnet), a novel architecture enhancing Transformer-based models by incorporating learnable radiomic features. This approach integrates dynamically adaptive Gabor and Laplacian of Gaussian (LoG) filters to capture texture, edge, and boundary information, enhancing the feature representation processed by the Transformer model. Our method uniquely combines the long-range dependency modeling of Transformers with the texture analysis capabilities of Gabor and LoG features. Evaluated on the Synapse multi-organ and ACDC cardiac segmentation datasets, GLoG-CSUnet demonstrates significant improvements over state-of-the-art models, achieving a 1.14% increase in Dice score for Synapse and 0.99% for ACDC, with minimal computational overhead (only 15 and 30 additional parameters, respectively). GLoG-CSUnet's flexible design allows integration with various base models, offering a promising approach for incorporating radiomics-inspired feature extraction in Transformer architectures for medical image analysis. The code implementation is available on GitHub at: https://github.com/HAAIL/GLoG-CSUnet.
Hybrid Student-Teacher Large Language Model Refinement for Cancer Toxicity Symptom Extraction
Aug 08, 2024


Abstract:Large Language Models (LLMs) offer significant potential for clinical symptom extraction, but their deployment in healthcare settings is constrained by privacy concerns, computational limitations, and operational costs. This study investigates the optimization of compact LLMs for cancer toxicity symptom extraction using a novel iterative refinement approach. We employ a student-teacher architecture, utilizing Zephyr-7b-beta and Phi3-mini-128 as student models and GPT-4o as the teacher, to dynamically select between prompt refinement, Retrieval-Augmented Generation (RAG), and fine-tuning strategies. Our experiments on 294 clinical notes covering 12 post-radiotherapy toxicity symptoms demonstrate the effectiveness of this approach. The RAG method proved most efficient, improving average accuracy scores from 0.32 to 0.73 for Zephyr-7b-beta and from 0.40 to 0.87 for Phi3-mini-128 during refinement. In the test set, both models showed an approximate 0.20 increase in accuracy across symptoms. Notably, this improvement was achieved at a cost 45 times lower than GPT-4o for Zephyr and 79 times lower for Phi-3. These results highlight the potential of iterative refinement techniques in enhancing the capabilities of compact LLMs for clinical applications, offering a balance between performance, cost-effectiveness, and privacy preservation in healthcare settings.
Iterative Prompt Refinement for Radiation Oncology Symptom Extraction Using Teacher-Student Large Language Models
Feb 06, 2024Abstract:This study introduces a novel teacher-student architecture utilizing Large Language Models (LLMs) to improve prostate cancer radiotherapy symptom extraction from clinical notes. Mixtral, the student model, initially extracts symptoms, followed by GPT-4, the teacher model, which refines prompts based on Mixtral's performance. This iterative process involved 294 single symptom clinical notes across 12 symptoms, with up to 16 rounds of refinement per epoch. Results showed significant improvements in extracting symptoms from both single and multi-symptom notes. For 59 single symptom notes, accuracy increased from 0.51 to 0.71, precision from 0.52 to 0.82, recall from 0.52 to 0.72, and F1 score from 0.49 to 0.73. In 375 multi-symptom notes, accuracy rose from 0.24 to 0.43, precision from 0.6 to 0.76, recall from 0.24 to 0.43, and F1 score from 0.20 to 0.44. These results demonstrate the effectiveness of advanced prompt engineering in LLMs for radiation oncology use.
An Introduction to Natural Language Processing Techniques and Framework for Clinical Implementation in Radiation Oncology
Nov 08, 2023

Abstract:Natural Language Processing (NLP) is a key technique for developing Medical Artificial Intelligence (AI) systems that leverage Electronic Health Record (EHR) data to build diagnostic and prognostic models. NLP enables the conversion of unstructured clinical text into structured data that can be fed into AI algorithms. The emergence of the transformer architecture and large language models (LLMs) has led to remarkable advances in NLP for various healthcare tasks, such as entity recognition, relation extraction, sentence similarity, text summarization, and question answering. In this article, we review the major technical innovations that underpin modern NLP models and present state-of-the-art NLP applications that employ LLMs in radiation oncology research. However, these LLMs are prone to many errors such as hallucinations, biases, and ethical violations, which necessitate rigorous evaluation and validation before clinical deployment. As such, we propose a comprehensive framework for assessing the NLP models based on their purpose and clinical fit, technical performance, bias and trust, legal and ethical implications, and quality assurance, prior to implementation in clinical radiation oncology. Our article aims to provide guidance and insights for researchers and clinicians who are interested in developing and using NLP models in clinical radiation oncology.
FocalUNETR: A Focal Transformer for Boundary-aware Segmentation of CT Images
Oct 06, 2022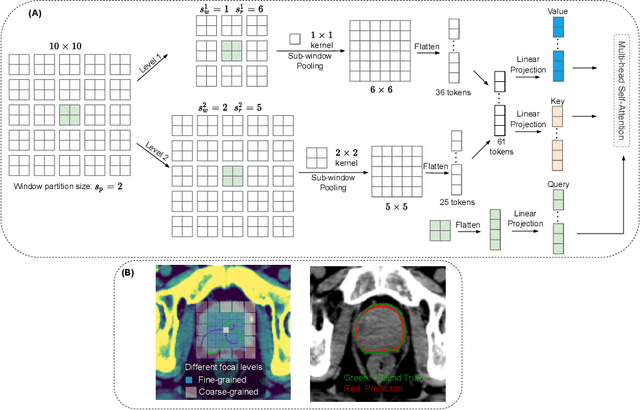
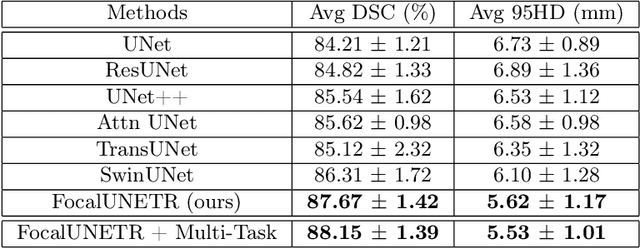
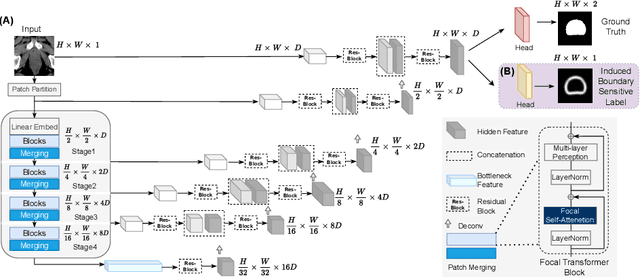

Abstract:Computed Tomography (CT) based precise prostate segmentation for treatment planning is challenging due to (1) the unclear boundary of prostate derived from CTs poor soft tissue contrast, and (2) the limitation of convolutional neural network based models in capturing long-range global context. Here we propose a focal transformer based image segmentation architecture to effectively and efficiently extract local visual features and global context from CT images. Furthermore, we design a main segmentation task and an auxiliary boundary-induced label regression task as regularization to simultaneously optimize segmentation results and mitigate the unclear boundary effect, particularly in unseen data set. Extensive experiments on a large data set of 400 prostate CT scans demonstrate the superior performance of our focal transformer to the competing methods on the prostate segmentation task.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge