Mirza Faisal Beg
School of Engineering Science, Simon Fraser University, Canada
Automated Body Composition Analysis Using DAFS Express on 2D MRI Slices at L3 Vertebral Level
Sep 11, 2024
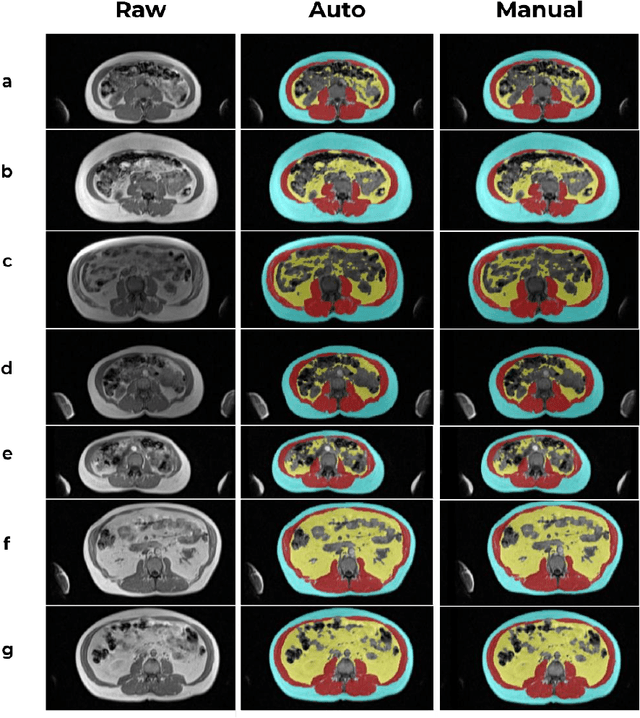
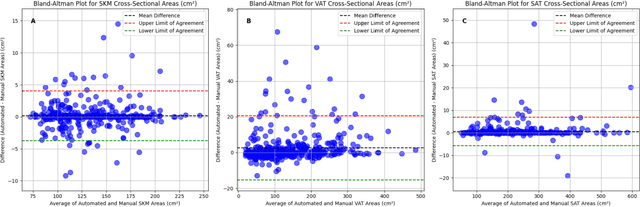
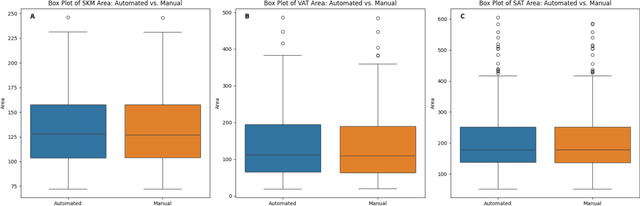
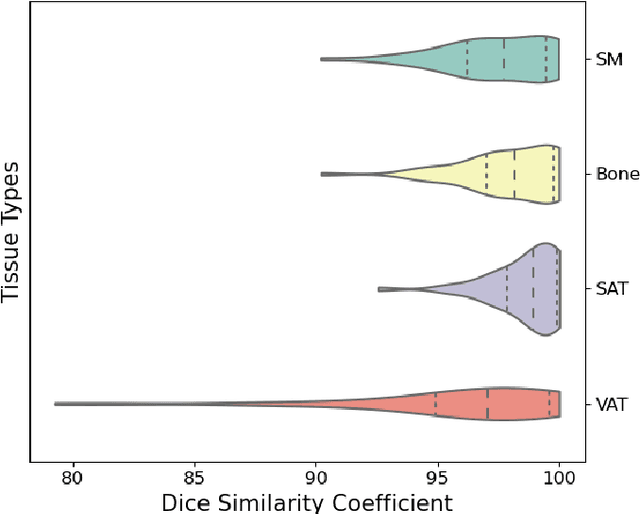
Abstract:Body composition analysis is vital in assessing health conditions such as obesity, sarcopenia, and metabolic syndromes. MRI provides detailed images of skeletal muscle (SKM), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT), but their manual segmentation is labor-intensive and limits clinical applicability. This study validates an automated tool for MRI-based 2D body composition analysis- (Data Analysis Facilitation Suite (DAFS) Express), comparing its automated measurements with expert manual segmentations using UK Biobank data. A cohort of 399 participants from the UK Biobank dataset was selected, yielding 423 single L3 slices for analysis. DAFS Express performed automated segmentations of SKM, VAT, and SAT, which were then manually corrected by expert raters for validation. Evaluation metrics included Jaccard coefficients, Dice scores, Intraclass Correlation Coefficients (ICCs), and Bland-Altman Plots to assess segmentation agreement and reliability. High agreements were observed between automated and manual segmentations with mean Jaccard scores: SKM 99.03%, VAT 95.25%, and SAT 99.57%; and mean Dice scores: SKM 99.51%, VAT 97.41%, and SAT 99.78%. Cross-sectional area comparisons showed consistent measurements with automated methods closely matching manual measurements for SKM and SAT, and slightly higher values for VAT (SKM: Auto 132.51 cm^2, Manual 132.36 cm^2; VAT: Auto 137.07 cm^2, Manual 134.46 cm^2; SAT: Auto 203.39 cm^2, Manual 202.85 cm^2). ICCs confirmed strong reliability (SKM: 0.998, VAT: 0.994, SAT: 0.994). Bland-Altman plots revealed minimal biases, and boxplots illustrated distribution similarities across SKM, VAT, and SAT areas. On average DAFS Express took 18 seconds per DICOM. This underscores its potential to streamline image analysis processes in research and clinical settings, enhancing diagnostic accuracy and efficiency.
Segmentation-guided Domain Adaptation and Data Harmonization of Multi-device Retinal Optical Coherence Tomography using Cycle-Consistent Generative Adversarial Networks
Aug 31, 2022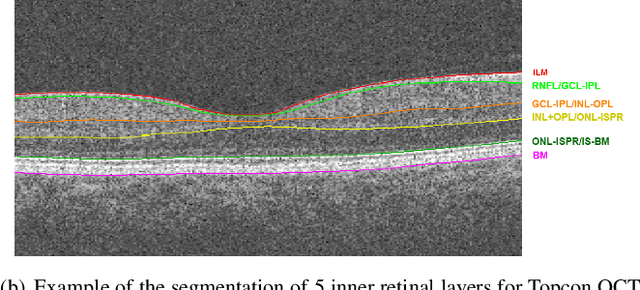
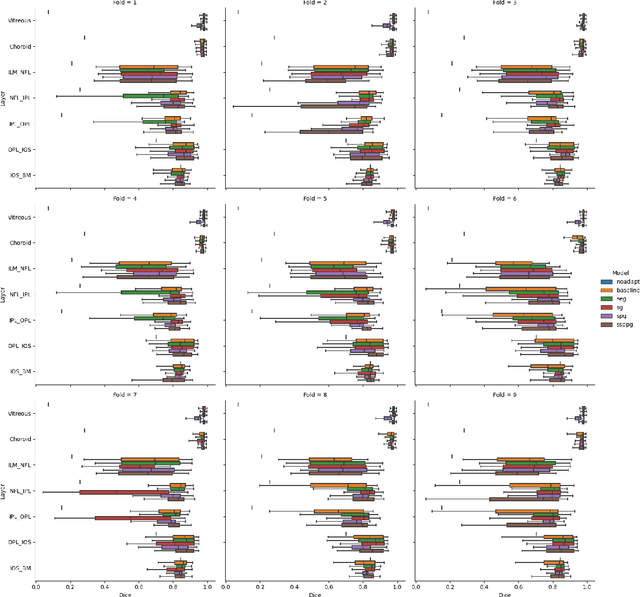
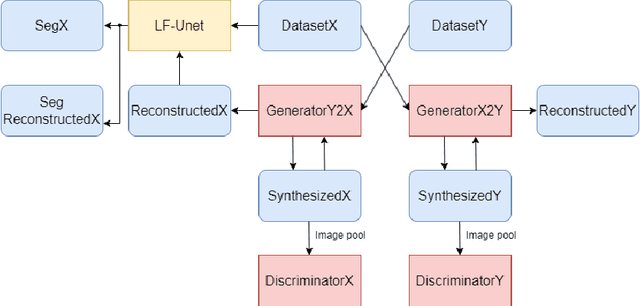
Abstract:Optical Coherence Tomography(OCT) is a non-invasive technique capturing cross-sectional area of the retina in micro-meter resolutions. It has been widely used as a auxiliary imaging reference to detect eye-related pathology and predict longitudinal progression of the disease characteristics. Retina layer segmentation is one of the crucial feature extraction techniques, where the variations of retinal layer thicknesses and the retinal layer deformation due to the presence of the fluid are highly correlated with multiple epidemic eye diseases like Diabetic Retinopathy(DR) and Age-related Macular Degeneration (AMD). However, these images are acquired from different devices, which have different intensity distribution, or in other words, belong to different imaging domains. This paper proposes a segmentation-guided domain-adaptation method to adapt images from multiple devices into single image domain, where the state-of-art pre-trained segmentation model is available. It avoids the time consumption of manual labelling for the upcoming new dataset and the re-training of the existing network. The semantic consistency and global feature consistency of the network will minimize the hallucination effect that many researchers reported regarding Cycle-Consistent Generative Adversarial Networks(CycleGAN) architecture.
Predicting Time-to-conversion for Dementia of Alzheimer's Type using Multi-modal Deep Survival Analysis
May 02, 2022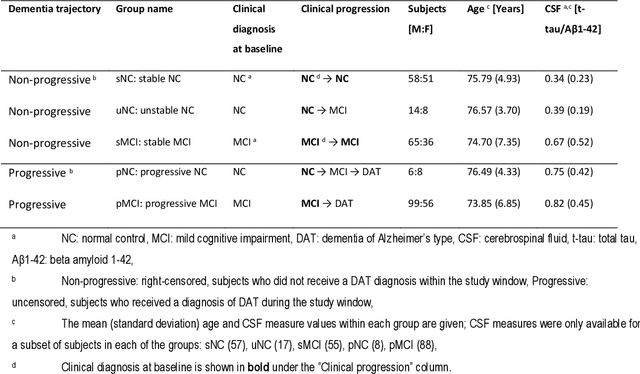
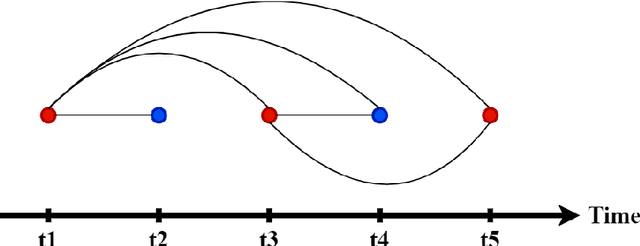
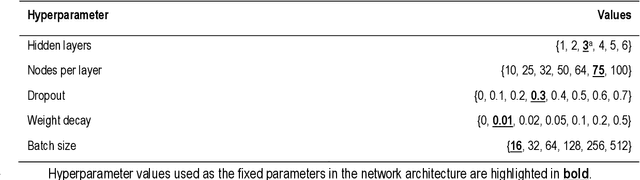
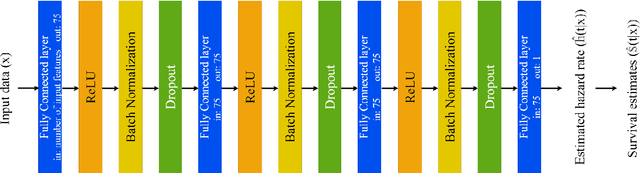
Abstract:Dementia of Alzheimer's Type (DAT) is a complex disorder influenced by numerous factors, but it is unclear how each factor contributes to disease progression. An in-depth examination of these factors may yield an accurate estimate of time-to-conversion to DAT for patients at various disease stages. We used 401 subjects with 63 features from MRI, genetic, and CDC (Cognitive tests, Demographic, and CSF) data modalities in the Alzheimer's Disease Neuroimaging Initiative (ADNI) database. We used a deep learning-based survival analysis model that extends the classic Cox regression model to predict time-to-conversion to DAT. Our findings showed that genetic features contributed the least to survival analysis, while CDC features contributed the most. Combining MRI and genetic features improved survival prediction over using either modality alone, but adding CDC to any combination of features only worked as well as using only CDC features. Consequently, our study demonstrated that using the current clinical procedure, which includes gathering cognitive test results, can outperform survival analysis results produced using costly genetic or CSF data.
Machine Learning Based Multimodal Neuroimaging Genomics Dementia Score for Predicting Future Conversion to Alzheimer's Disease
Mar 11, 2022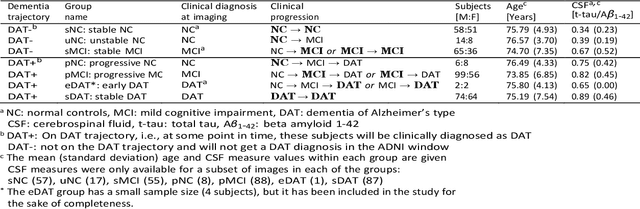
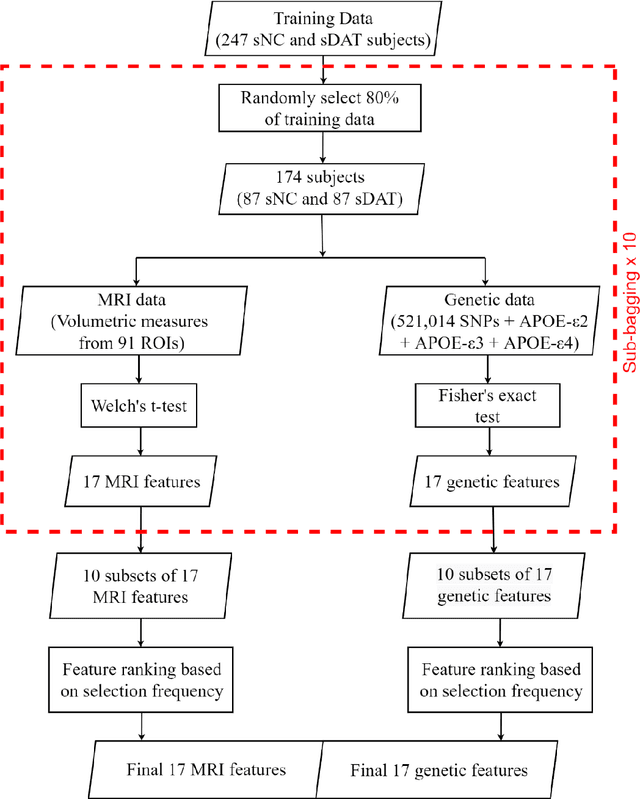
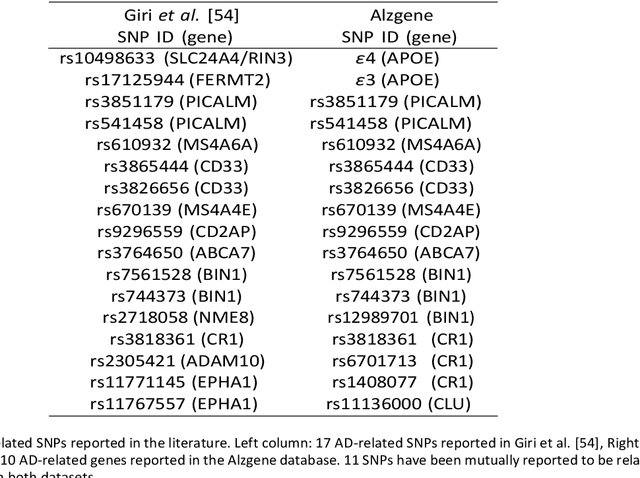
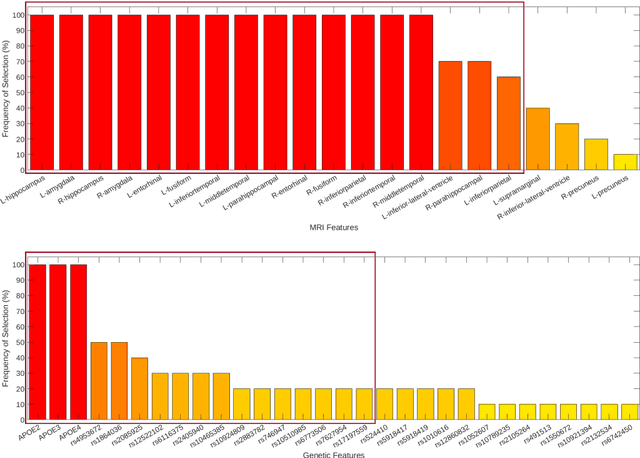
Abstract:Background: The increasing availability of databases containing both magnetic resonance imaging (MRI) and genetic data allows researchers to utilize multimodal data to better understand the characteristics of dementia of Alzheimer's type (DAT). Objective: The goal of this study was to develop and analyze novel biomarkers that can help predict the development and progression of DAT. Methods: We used feature selection and ensemble learning classifier to develop an image/genotype-based DAT score that represents a subject's likelihood of developing DAT in the future. Three feature types were used: MRI only, genetic only, and combined multimodal data. We used a novel data stratification method to better represent different stages of DAT. Using a pre-defined 0.5 threshold on DAT scores, we predicted whether or not a subject would develop DAT in the future. Results: Our results on Alzheimer's Disease Neuroimaging Initiative (ADNI) database showed that dementia scores using genetic data could better predict future DAT progression for currently normal control subjects (Accuracy=0.857) compared to MRI (Accuracy=0.143), while MRI can better characterize subjects with stable mild cognitive impairment (Accuracy=0.614) compared to genetics (Accuracy=0.356). Combining MRI and genetic data showed improved classification performance in the remaining stratified groups. Conclusion: MRI and genetic data can contribute to DAT prediction in different ways. MRI data reflects anatomical changes in the brain, while genetic data can detect the risk of DAT progression prior to the symptomatic onset. Combining information from multimodal data in the right way can improve prediction performance.
Differential Diagnosis of Frontotemporal Dementia and Alzheimer's Disease using Generative Adversarial Network
Sep 29, 2021



Abstract:Frontotemporal dementia and Alzheimer's disease are two common forms of dementia and are easily misdiagnosed as each other due to their similar pattern of clinical symptoms. Differentiating between the two dementia types is crucial for determining disease-specific intervention and treatment. Recent development of Deep-learning-based approaches in the field of medical image computing are delivering some of the best performance for many binary classification tasks, although its application in differential diagnosis, such as neuroimage-based differentiation for multiple types of dementia, has not been explored. In this study, a novel framework was proposed by using the Generative Adversarial Network technique to distinguish FTD, AD and normal control subjects, using volumetric features extracted at coarse-to-fine structural scales from Magnetic Resonance Imaging scans. Experiments of 10-folds cross-validation on 1,954 images achieved high accuracy. With the proposed framework, we have demonstrated that the combination of multi-scale structural features and synthetic data augmentation based on generative adversarial network can improve the performance of challenging tasks such as differentiating Dementia sub-types.
Domain Adaptation via CycleGAN for Retina Segmentation in Optical Coherence Tomography
Jul 06, 2021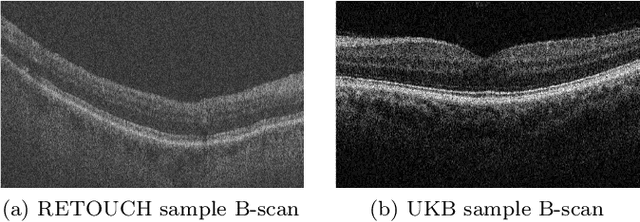
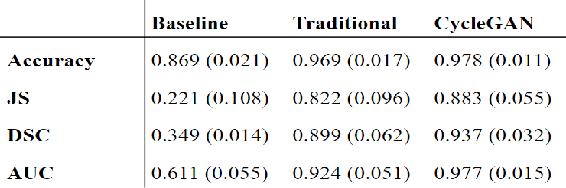
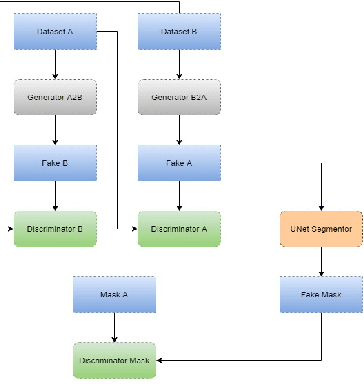
Abstract:With the FDA approval of Artificial Intelligence (AI) for point-of-care clinical diagnoses, model generalizability is of the utmost importance as clinical decision-making must be domain-agnostic. A method of tackling the problem is to increase the dataset to include images from a multitude of domains; while this technique is ideal, the security requirements of medical data is a major limitation. Additionally, researchers with developed tools benefit from the addition of open-sourced data, but are limited by the difference in domains. Herewith, we investigated the implementation of a Cycle-Consistent Generative Adversarial Networks (CycleGAN) for the domain adaptation of Optical Coherence Tomography (OCT) volumes. This study was done in collaboration with the Biomedical Optics Research Group and Functional & Anatomical Imaging & Shape Analysis Lab at Simon Fraser University. In this study, we investigated a learning-based approach of adapting the domain of a publicly available dataset, UK Biobank dataset (UKB). To evaluate the performance of domain adaptation, we utilized pre-existing retinal layer segmentation tools developed on a different set of RETOUCH OCT data. This study provides insight on state-of-the-art tools for domain adaptation compared to traditional processing techniques as well as a pipeline for adapting publicly available retinal data to the domains previously used by our collaborators.
Comprehensive Validation of Automated Whole Body Skeletal Muscle, Adipose Tissue, and Bone Segmentation from 3D CT images for Body Composition Analysis: Towards Extended Body Composition
Jun 03, 2021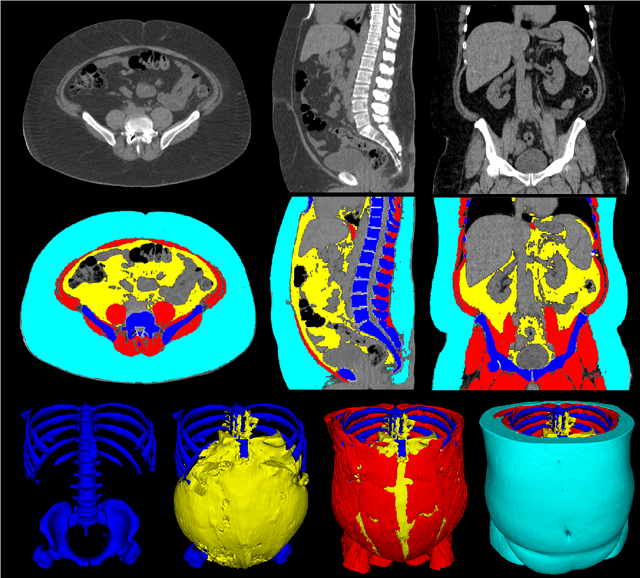


Abstract:The latest advances in computer-assisted precision medicine are making it feasible to move from population-wide models that are useful to discover aggregate patterns that hold for group-based analysis to patient-specific models that can drive patient-specific decisions with regard to treatment choices, and predictions of outcomes of treatment. Body Composition is recognized as an important driver and risk factor for a wide variety of diseases, as well as a predictor of individual patient-specific clinical outcomes to treatment choices or surgical interventions. 3D CT images are routinely acquired in the oncological worklows and deliver accurate rendering of internal anatomy and therefore can be used opportunistically to assess the amount of skeletal muscle and adipose tissue compartments. Powerful tools of artificial intelligence such as deep learning are making it feasible now to segment the entire 3D image and generate accurate measurements of all internal anatomy. These will enable the overcoming of the severe bottleneck that existed previously, namely, the need for manual segmentation, which was prohibitive to scale to the hundreds of 2D axial slices that made up a 3D volumetric image. Automated tools such as presented here will now enable harvesting whole-body measurements from 3D CT or MRI images, leading to a new era of discovery of the drivers of various diseases based on individual tissue, organ volume, shape, and functional status. These measurements were hitherto unavailable thereby limiting the field to a very small and limited subset. These discoveries and the potential to perform individual image segmentation with high speed and accuracy are likely to lead to the incorporation of these 3D measures into individual specific treatment planning models related to nutrition, aging, chemotoxicity, surgery and survival after the onset of a major disease such as cancer.
Microvasculature Segmentation and Inter-capillary Area Quantification of the Deep Vascular Complex using Transfer Learning
Mar 19, 2020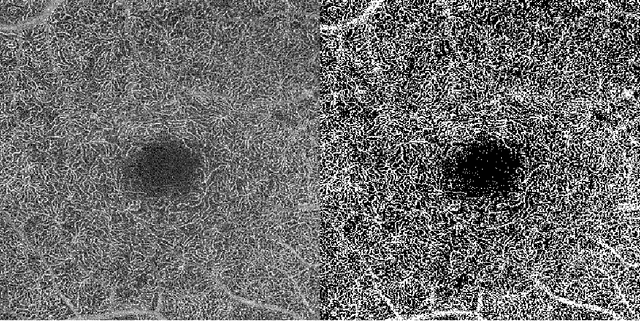

Abstract:Purpose: Optical Coherence Tomography Angiography (OCT-A) permits visualization of the changes to the retinal circulation due to diabetic retinopathy (DR), a microvascular complication of diabetes. We demonstrate accurate segmentation of the vascular morphology for the superficial capillary plexus and deep vascular complex (SCP and DVC) using a convolutional neural network (CNN) for quantitative analysis. Methods: Retinal OCT-A with a 6x6mm field of view (FOV) were acquired using a Zeiss PlexElite. Multiple-volume acquisition and averaging enhanced the vessel network contrast used for training the CNN. We used transfer learning from a CNN trained on 76 images from smaller FOVs of the SCP acquired using different OCT systems. Quantitative analysis of perfusion was performed on the automated vessel segmentations in representative patients with DR. Results: The automated segmentations of the OCT-A images maintained the hierarchical branching and lobular morphologies of the SCP and DVC, respectively. The network segmented the SCP with an accuracy of 0.8599, and a Dice index of 0.8618. For the DVC, the accuracy was 0.7986, and the Dice index was 0.8139. The inter-rater comparisons for the SCP had an accuracy and Dice index of 0.8300 and 0.6700, respectively, and 0.6874 and 0.7416 for the DVC. Conclusions: Transfer learning reduces the amount of manually-annotated images required, while producing high quality automatic segmentations of the SCP and DVC. Using high quality training data preserves the characteristic appearance of the capillary networks in each layer. Translational Relevance: Accurate retinal microvasculature segmentation with the CNN results in improved perfusion analysis in diabetic retinopathy.
Cascaded Deep Neural Networks for Retinal Layer Segmentation of Optical Coherence Tomography with Fluid Presence
Dec 07, 2019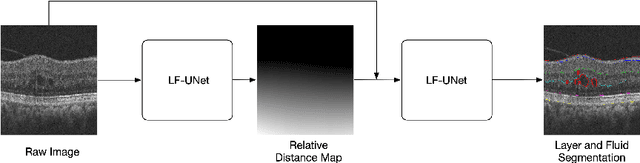
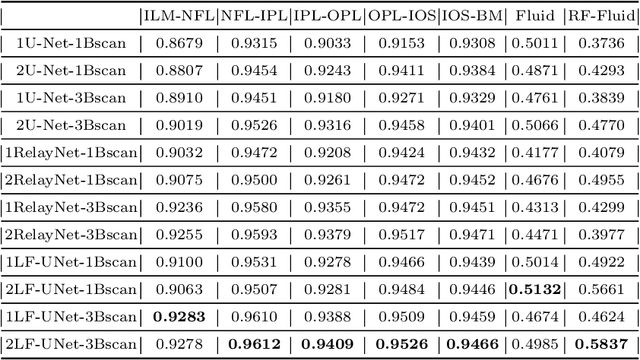
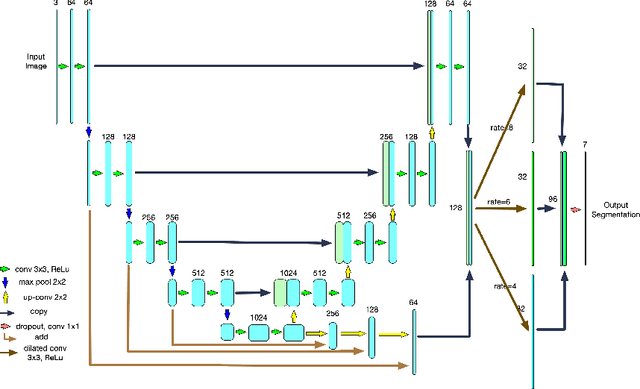
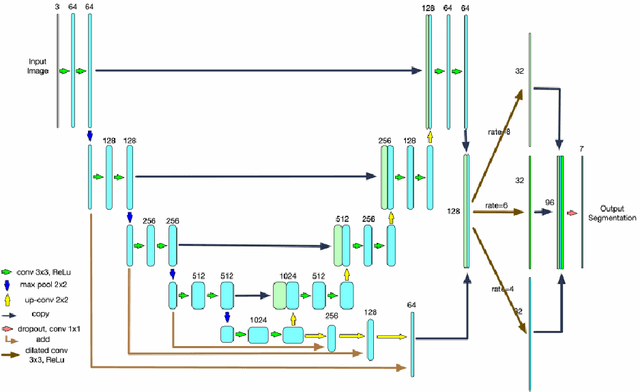
Abstract:Optical coherence tomography (OCT) is a non-invasive imaging technology which can provide micrometer-resolution cross-sectional images of the inner structures of the eye. It is widely used for the diagnosis of ophthalmic diseases with retinal alteration, such as layer deformation and fluid accumulation. In this paper, a novel framework was proposed to segment retinal layers with fluid presence. The main contribution of this study is two folds: 1) we developed a cascaded network framework to incorporate the prior structural knowledge; 2) we proposed a novel deep neural network based on U-Net and fully convolutional network, termed LF-UNet. Cross validation experiments proved that the proposed LF-UNet has superior performance comparing with the state-of-the-art methods, and incorporating the relative distance map structural prior information could further improve the performance regardless the network.
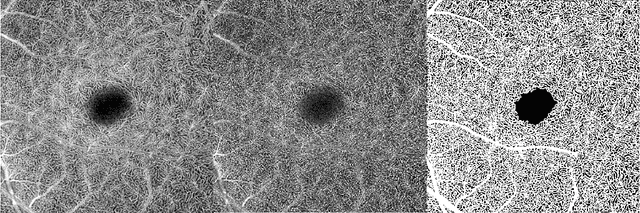
Deep learning vessel segmentation and quantification of the foveal avascular zone using commercial and prototype OCT-A platforms
Sep 25, 2019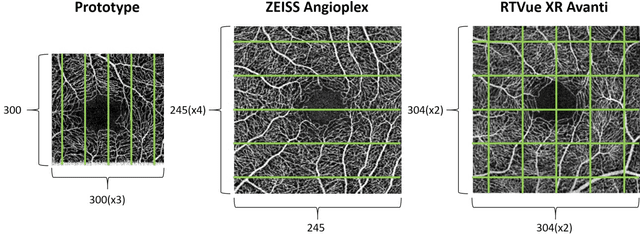
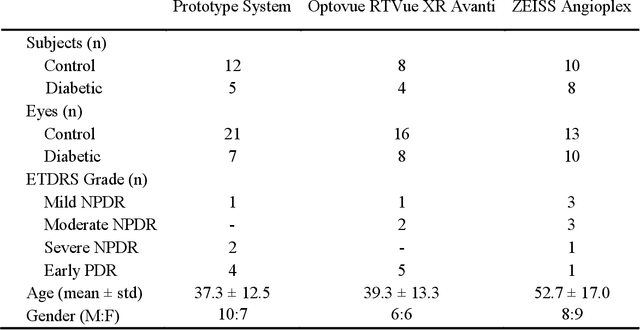

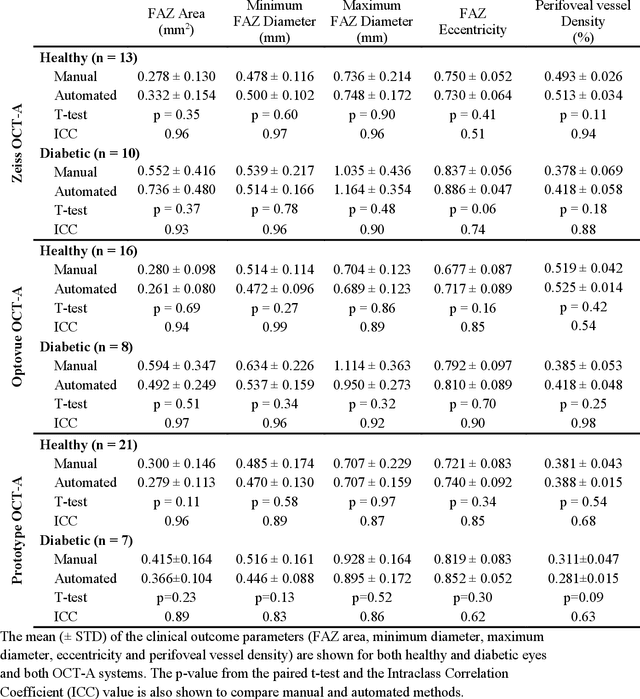
Abstract:Automatic quantification of perifoveal vessel densities in optical coherence tomography angiography (OCT-A) images face challenges such as variable intra- and inter-image signal to noise ratios, projection artefacts from outer vasculature layers, and motion artefacts. This study demonstrates the utility of deep neural networks for automatic quantification of foveal avascular zone (FAZ) parameters and perifoveal vessel density of OCT-A images in healthy and diabetic eyes. OCT-A images of the foveal region were acquired using three OCT-A systems: a 1060nm Swept Source (SS)-OCT prototype, RTVue XR Avanti (Optovue Inc., Fremont, CA), and the ZEISS Angioplex (Carl Zeiss Meditec, Dublin, CA). Automated segmentation was then performed using a deep neural network. Four FAZ morphometric parameters (area, min/max diameter, and eccentricity) and perifoveal vessel density were used as outcome measures. The accuracy, sensitivity and specificity of the DNN vessel segmentations were comparable across all three device platforms. No significant difference between the means of the measurements from automated and manual segmentations were found for any of the outcome measures on any system. The intraclass correlation coefficient (ICC) was also good (> 0.51) for all measurements. Automated deep learning vessel segmentation of OCT-A may be suitable for both commercial and research purposes for better quantification of the retinal circulation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge