Gavin Xu
Segmentation-guided Domain Adaptation and Data Harmonization of Multi-device Retinal Optical Coherence Tomography using Cycle-Consistent Generative Adversarial Networks
Aug 31, 2022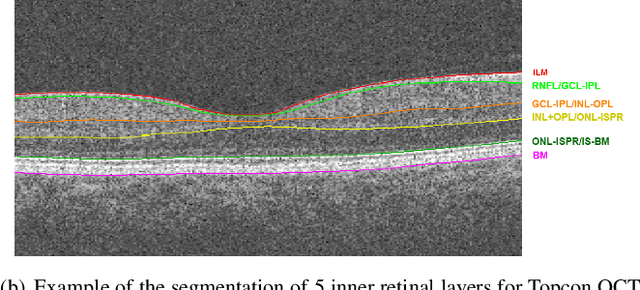
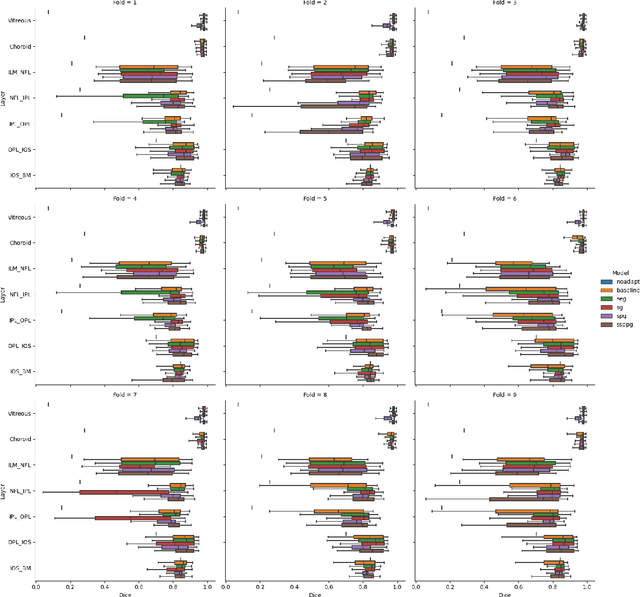
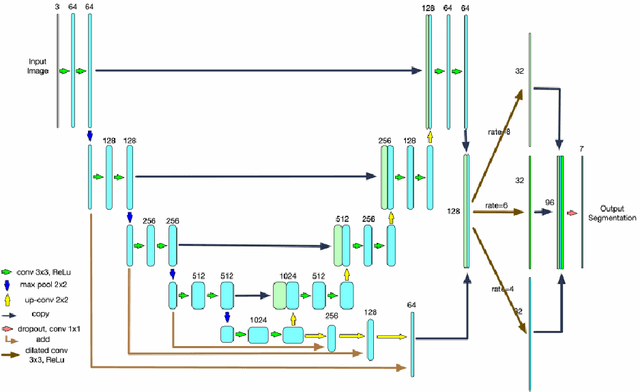
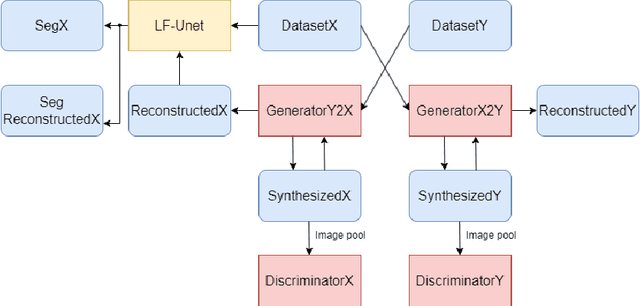
Abstract:Optical Coherence Tomography(OCT) is a non-invasive technique capturing cross-sectional area of the retina in micro-meter resolutions. It has been widely used as a auxiliary imaging reference to detect eye-related pathology and predict longitudinal progression of the disease characteristics. Retina layer segmentation is one of the crucial feature extraction techniques, where the variations of retinal layer thicknesses and the retinal layer deformation due to the presence of the fluid are highly correlated with multiple epidemic eye diseases like Diabetic Retinopathy(DR) and Age-related Macular Degeneration (AMD). However, these images are acquired from different devices, which have different intensity distribution, or in other words, belong to different imaging domains. This paper proposes a segmentation-guided domain-adaptation method to adapt images from multiple devices into single image domain, where the state-of-art pre-trained segmentation model is available. It avoids the time consumption of manual labelling for the upcoming new dataset and the re-training of the existing network. The semantic consistency and global feature consistency of the network will minimize the hallucination effect that many researchers reported regarding Cycle-Consistent Generative Adversarial Networks(CycleGAN) architecture.
Domain Adaptation via CycleGAN for Retina Segmentation in Optical Coherence Tomography
Jul 06, 2021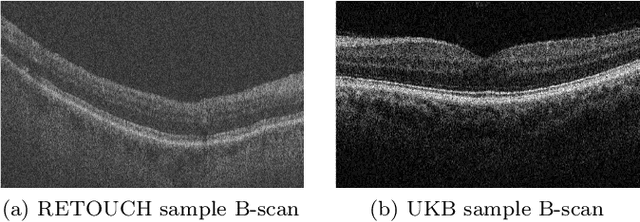
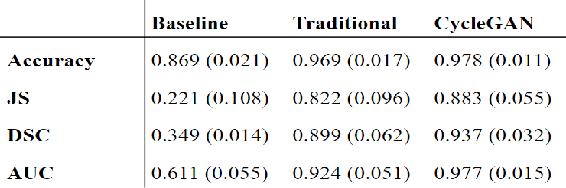
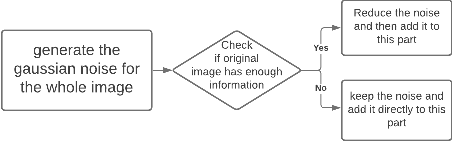
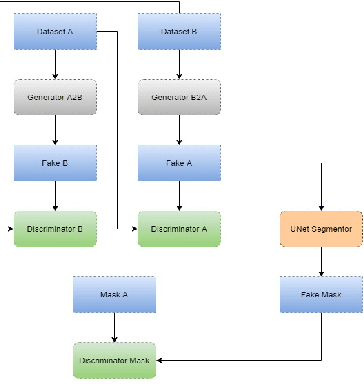
Abstract:With the FDA approval of Artificial Intelligence (AI) for point-of-care clinical diagnoses, model generalizability is of the utmost importance as clinical decision-making must be domain-agnostic. A method of tackling the problem is to increase the dataset to include images from a multitude of domains; while this technique is ideal, the security requirements of medical data is a major limitation. Additionally, researchers with developed tools benefit from the addition of open-sourced data, but are limited by the difference in domains. Herewith, we investigated the implementation of a Cycle-Consistent Generative Adversarial Networks (CycleGAN) for the domain adaptation of Optical Coherence Tomography (OCT) volumes. This study was done in collaboration with the Biomedical Optics Research Group and Functional & Anatomical Imaging & Shape Analysis Lab at Simon Fraser University. In this study, we investigated a learning-based approach of adapting the domain of a publicly available dataset, UK Biobank dataset (UKB). To evaluate the performance of domain adaptation, we utilized pre-existing retinal layer segmentation tools developed on a different set of RETOUCH OCT data. This study provides insight on state-of-the-art tools for domain adaptation compared to traditional processing techniques as well as a pipeline for adapting publicly available retinal data to the domains previously used by our collaborators.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge