Timothy T. Yu
Evaluating LLM Reasoning in the Operations Research Domain with ORQA
Dec 22, 2024

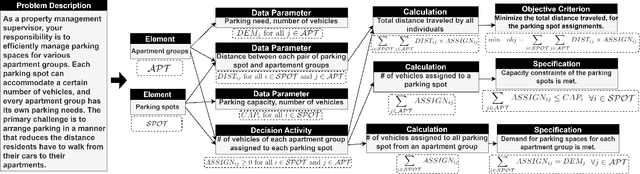
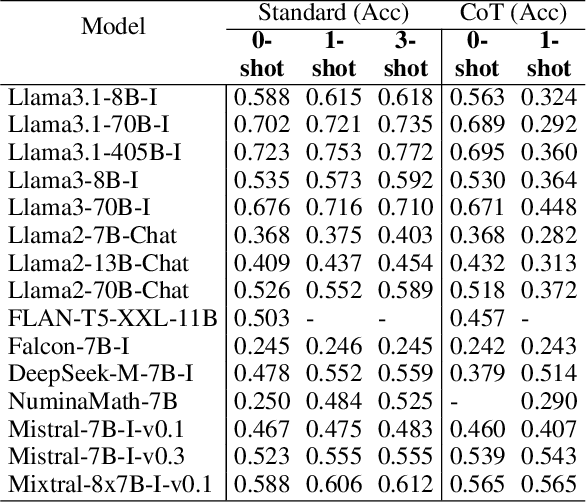
Abstract:In this paper, we introduce and apply Operations Research Question Answering (ORQA), a new benchmark designed to assess the generalization capabilities of Large Language Models (LLMs) in the specialized technical domain of Operations Research (OR). This benchmark evaluates whether LLMs can emulate the knowledge and reasoning skills of OR experts when confronted with diverse and complex optimization problems. The dataset, developed by OR experts, features real-world optimization problems that demand multistep reasoning to construct their mathematical models. Our evaluations of various open source LLMs, such as LLaMA 3.1, DeepSeek, and Mixtral, reveal their modest performance, highlighting a gap in their ability to generalize to specialized technical domains. This work contributes to the ongoing discourse on LLMs generalization capabilities, offering valuable insights for future research in this area. The dataset and evaluation code are publicly available.
NL4Opt Competition: Formulating Optimization Problems Based on Their Natural Language Descriptions
Mar 27, 2023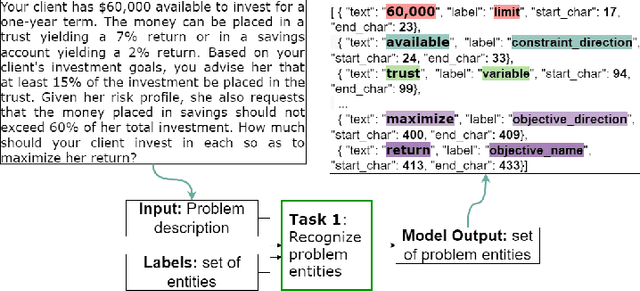
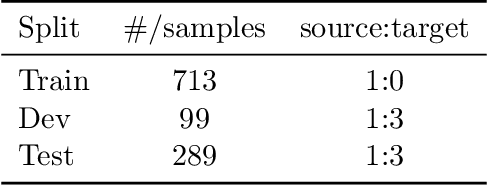
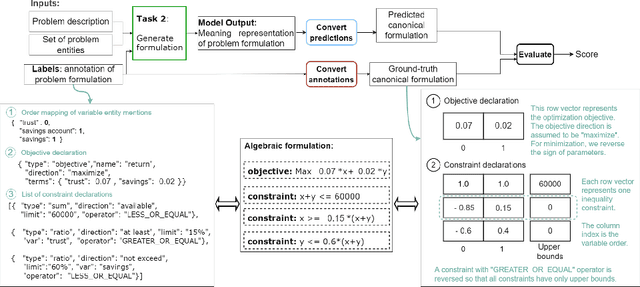
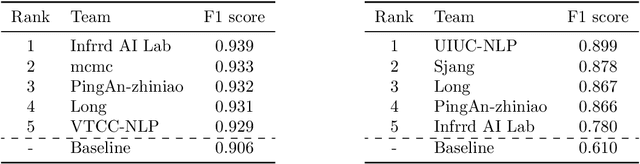
Abstract:The Natural Language for Optimization (NL4Opt) Competition was created to investigate methods of extracting the meaning and formulation of an optimization problem based on its text description. Specifically, the goal of the competition is to increase the accessibility and usability of optimization solvers by allowing non-experts to interface with them using natural language. We separate this challenging goal into two sub-tasks: (1) recognize and label the semantic entities that correspond to the components of the optimization problem; (2) generate a meaning representation (i.e., a logical form) of the problem from its detected problem entities. The first task aims to reduce ambiguity by detecting and tagging the entities of the optimization problems. The second task creates an intermediate representation of the linear programming (LP) problem that is converted into a format that can be used by commercial solvers. In this report, we present the LP word problem dataset and shared tasks for the NeurIPS 2022 competition. Furthermore, we investigate and compare the performance of the ChatGPT large language model against the winning solutions. Through this competition, we hope to bring interest towards the development of novel machine learning applications and datasets for optimization modeling.
Spectral Bandwidth Recovery of Optical Coherence Tomography Images using Deep Learning
Jan 02, 2023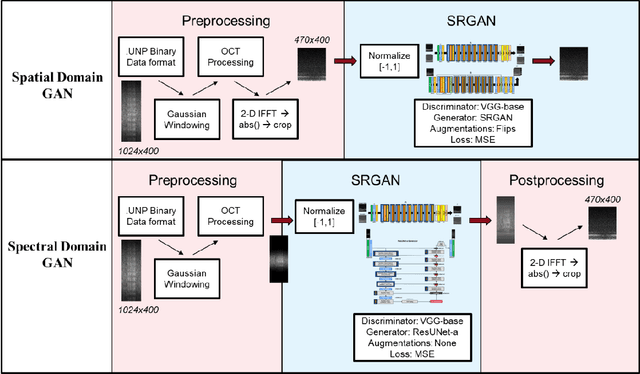
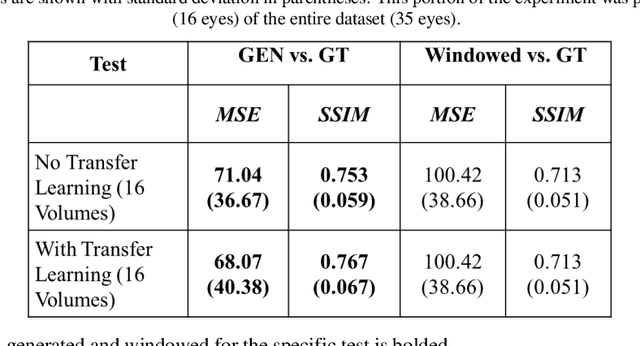
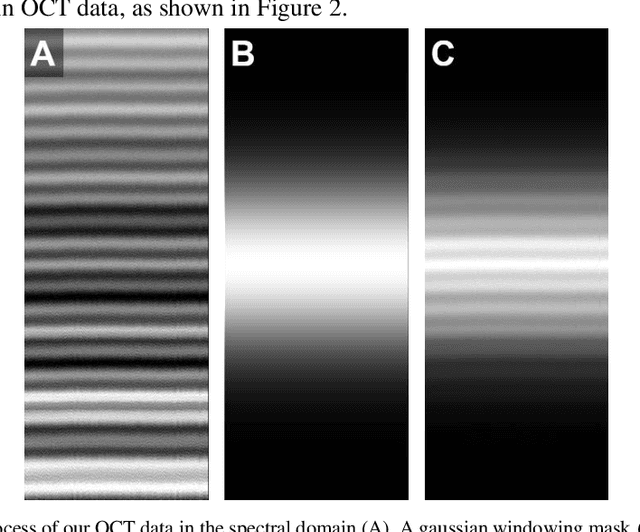
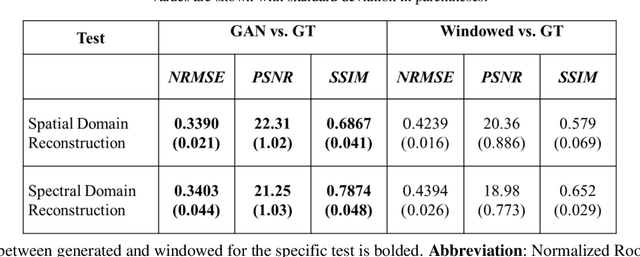
Abstract:Optical coherence tomography (OCT) captures cross-sectional data and is used for the screening, monitoring, and treatment planning of retinal diseases. Technological developments to increase the speed of acquisition often results in systems with a narrower spectral bandwidth, and hence a lower axial resolution. Traditionally, image-processing-based techniques have been utilized to reconstruct subsampled OCT data and more recently, deep-learning-based methods have been explored. In this study, we simulate reduced axial scan (A-scan) resolution by Gaussian windowing in the spectral domain and investigate the use of a learning-based approach for image feature reconstruction. In anticipation of the reduced resolution that accompanies wide-field OCT systems, we build upon super-resolution techniques to explore methods to better aid clinicians in their decision-making to improve patient outcomes, by reconstructing lost features using a pixel-to-pixel approach with an altered super-resolution generative adversarial network (SRGAN) architecture.
Augmenting Operations Research with Auto-Formulation of Optimization Models from Problem Descriptions
Oct 11, 2022



Abstract:We describe an augmented intelligence system for simplifying and enhancing the modeling experience for operations research. Using this system, the user receives a suggested formulation of an optimization problem based on its description. To facilitate this process, we build an intuitive user interface system that enables the users to validate and edit the suggestions. We investigate controlled generation techniques to obtain an automatic suggestion of formulation. Then, we evaluate their effectiveness with a newly created dataset of linear programming problems drawn from various application domains.
Domain Adaptation via CycleGAN for Retina Segmentation in Optical Coherence Tomography
Jul 06, 2021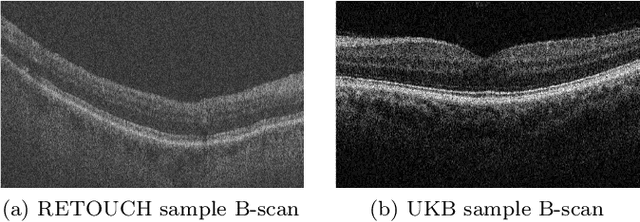
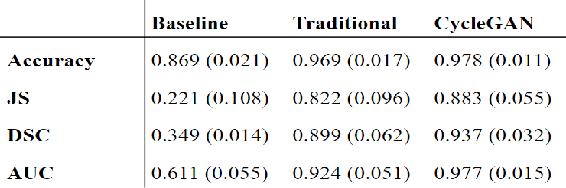
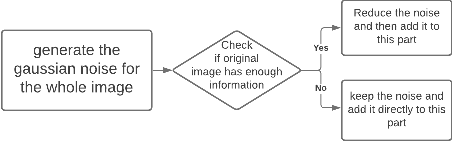
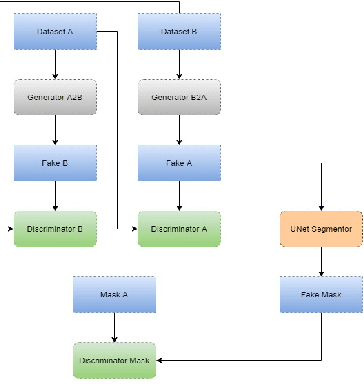
Abstract:With the FDA approval of Artificial Intelligence (AI) for point-of-care clinical diagnoses, model generalizability is of the utmost importance as clinical decision-making must be domain-agnostic. A method of tackling the problem is to increase the dataset to include images from a multitude of domains; while this technique is ideal, the security requirements of medical data is a major limitation. Additionally, researchers with developed tools benefit from the addition of open-sourced data, but are limited by the difference in domains. Herewith, we investigated the implementation of a Cycle-Consistent Generative Adversarial Networks (CycleGAN) for the domain adaptation of Optical Coherence Tomography (OCT) volumes. This study was done in collaboration with the Biomedical Optics Research Group and Functional & Anatomical Imaging & Shape Analysis Lab at Simon Fraser University. In this study, we investigated a learning-based approach of adapting the domain of a publicly available dataset, UK Biobank dataset (UKB). To evaluate the performance of domain adaptation, we utilized pre-existing retinal layer segmentation tools developed on a different set of RETOUCH OCT data. This study provides insight on state-of-the-art tools for domain adaptation compared to traditional processing techniques as well as a pipeline for adapting publicly available retinal data to the domains previously used by our collaborators.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge