Mahsa Shoaran
BrainDistill: Implantable Motor Decoding with Task-Specific Knowledge Distillation
Jan 24, 2026Abstract:Transformer-based neural decoders with large parameter counts, pre-trained on large-scale datasets, have recently outperformed classical machine learning models and small neural networks on brain-computer interface (BCI) tasks. However, their large parameter counts and high computational demands hinder deployment in power-constrained implantable systems. To address this challenge, we introduce BrainDistill, a novel implantable motor decoding pipeline that integrates an implantable neural decoder (IND) with a task-specific knowledge distillation (TSKD) framework. Unlike standard feature distillation methods that attempt to preserve teacher representations in full, TSKD explicitly prioritizes features critical for decoding through supervised projection. Across multiple neural datasets, IND consistently outperforms prior neural decoders on motor decoding tasks, while its TSKD-distilled variant further surpasses alternative distillation methods in few-shot calibration settings. Finally, we present a quantization-aware training scheme that enables integer-only inference with activation clipping ranges learned during training. The quantized IND enables deployment under the strict power constraints of implantable BCIs with minimal performance loss.
rETF-semiSL: Semi-Supervised Learning for Neural Collapse in Temporal Data
Aug 13, 2025Abstract:Deep neural networks for time series must capture complex temporal patterns, to effectively represent dynamic data. Self- and semi-supervised learning methods show promising results in pre-training large models, which -- when finetuned for classification -- often outperform their counterparts trained from scratch. Still, the choice of pretext training tasks is often heuristic and their transferability to downstream classification is not granted, thus we propose a novel semi-supervised pre-training strategy to enforce latent representations that satisfy the Neural Collapse phenomenon observed in optimally trained neural classifiers. We use a rotational equiangular tight frame-classifier and pseudo-labeling to pre-train deep encoders with few labeled samples. Furthermore, to effectively capture temporal dynamics while enforcing embedding separability, we integrate generative pretext tasks with our method, and we define a novel sequential augmentation strategy. We show that our method significantly outperforms previous pretext tasks when applied to LSTMs, transformers, and state-space models on three multivariate time series classification datasets. These results highlight the benefit of aligning pre-training objectives with theoretically grounded embedding geometry.
Machine-Learning-Powered Neural Interfaces for Smart Prosthetics and Diagnostics
May 05, 2025Abstract:Advanced neural interfaces are transforming applications ranging from neuroscience research to diagnostic tools (for mental state recognition, tremor and seizure detection) as well as prosthetic devices (for motor and communication recovery). By integrating complex functions into miniaturized neural devices, these systems unlock significant opportunities for personalized assistive technologies and adaptive therapeutic interventions. Leveraging high-density neural recordings, on-site signal processing, and machine learning (ML), these interfaces extract critical features, identify disease neuro-markers, and enable accurate, low-latency neural decoding. This integration facilitates real-time interpretation of neural signals, adaptive modulation of brain activity, and efficient control of assistive devices. Moreover, the synergy between neural interfaces and ML has paved the way for self-sufficient, ubiquitous platforms capable of operating in diverse environments with minimal hardware costs and external dependencies. In this work, we review recent advancements in AI-driven decoding algorithms and energy-efficient System-on-Chip (SoC) platforms for next-generation miniaturized neural devices. These innovations highlight the potential for developing intelligent neural interfaces, addressing critical challenges in scalability, reliability, interpretability, and user adaptability.
MT-NAM: An Efficient and Adaptive Model for Epileptic Seizure Detection
Mar 11, 2025Abstract:Enhancing the accuracy and efficiency of machine learning algorithms employed in neural interface systems is crucial for advancing next-generation intelligent therapeutic devices. However, current systems often utilize basic machine learning models that do not fully exploit the natural structure of brain signals. Additionally, existing learning models used for neural signal processing often demonstrate low speed and efficiency during inference. To address these challenges, this study introduces Micro Tree-based NAM (MT-NAM), a distilled model based on the recently proposed Neural Additive Models (NAM). The MT-NAM achieves a remarkable 100$\times$ improvement in inference speed compared to standard NAM, without compromising accuracy. We evaluate our approach on the CHB-MIT scalp EEG dataset, which includes recordings from 24 patients with varying numbers of sessions and seizures. NAM achieves an 85.3\% window-based sensitivity and 95\% specificity. Interestingly, our proposed MT-NAM shows only a 2\% reduction in sensitivity compared to the original NAM. To regain this sensitivity, we utilize a test-time template adjuster (T3A) as an update mechanism, enabling our model to achieve higher sensitivity during test time by accommodating transient shifts in neural signals. With this online update approach, MT-NAM achieves the same sensitivity as the standard NAM while achieving approximately 50$\times$ acceleration in inference speed.
Linear Attention for Efficient Bidirectional Sequence Modeling
Feb 22, 2025Abstract:Transformers with linear attention enable fast and parallel training. Moreover, they can be formulated as Recurrent Neural Networks (RNNs), for efficient linear-time inference. While extensively evaluated in causal sequence modeling, they have yet to be extended to the bidirectional setting. This work introduces the LION framework, establishing new theoretical foundations for linear transformers in bidirectional sequence modeling. LION constructs a bidirectional RNN equivalent to full Linear Attention. This extends the benefits of linear transformers: parallel training, and efficient inference, into the bidirectional setting. Using LION, we cast three linear transformers to their bidirectional form: LION-LIT, the bidirectional variant corresponding to (Katharopoulos et al., 2020); LION-D, extending RetNet (Sun et al., 2023); and LION-S, a linear transformer with a stable selective mask inspired by selectivity of SSMs (Dao & Gu, 2024). Replacing the attention block with LION (-LIT, -D, -S) achieves performance on bidirectional tasks that approaches that of Transformers and State-Space Models (SSMs), while delivering significant improvements in training speed. Our implementation is available in http://github.com/LIONS-EPFL/LION.
Hardware-Efficient EMG Decoding for Next-Generation Hand Prostheses
May 31, 2024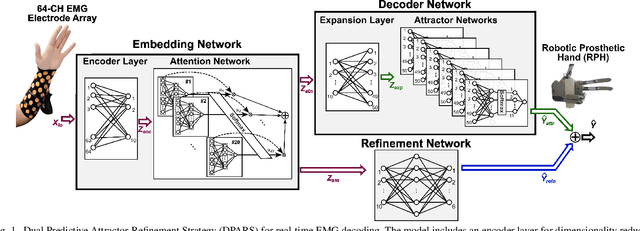
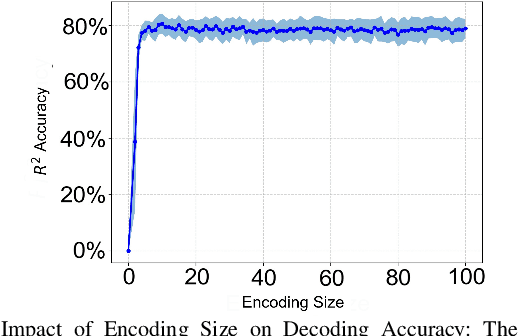
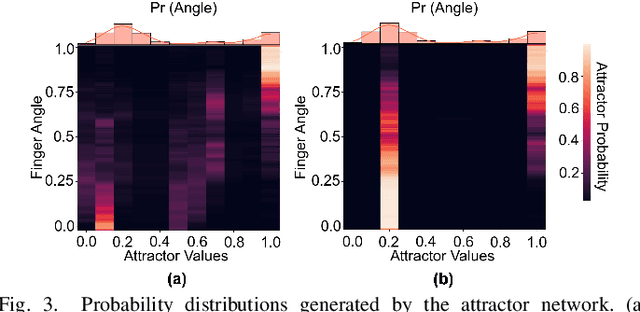
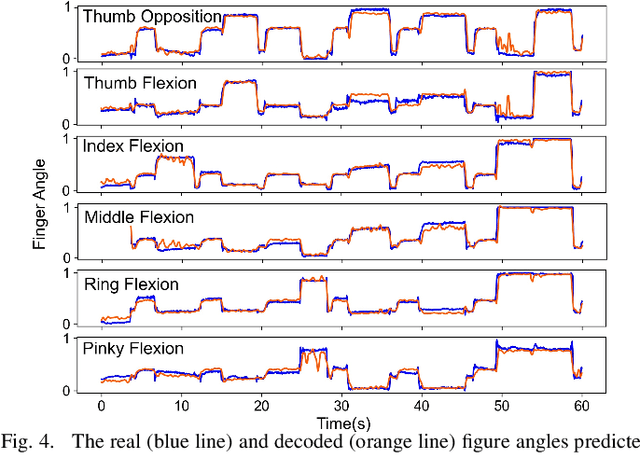
Abstract:Advancements in neural engineering have enabled the development of Robotic Prosthetic Hands (RPHs) aimed at restoring hand functionality. Current commercial RPHs offer limited control through basic on/off commands. Recent progresses in machine learning enable finger movement decoding with higher degrees of freedom, yet the high computational complexity of such models limits their application in portable devices. Future RPH designs must balance portability, low power consumption, and high decoding accuracy to be practical for individuals with disabilities. To this end, we introduce a novel attractor-based neural network to realize on-chip movement decoding for next-generation portable RPHs. The proposed architecture comprises an encoder, an attention layer, an attractor network, and a refinement regressor. We tested our model on four healthy subjects and achieved a decoding accuracy of 80.3%. Our proposed model is over 120 and 50 times more compact compared to state-of-the-art LSTM and CNN models, respectively, with comparable (or superior) decoding accuracy. Therefore, it exhibits minimal hardware complexity and can be effectively integrated as a System-on-Chip.
Intelligent Neural Interfaces: An Emerging Era in Neurotechnology
May 13, 2024Abstract:Integrating smart algorithms on neural devices presents significant opportunities for various brain disorders. In this paper, we review the latest advancements in the development of three categories of intelligent neural prostheses featuring embedded signal processing on the implantable or wearable device. These include: 1) Neural interfaces for closed-loop symptom tracking and responsive stimulation; 2) Neural interfaces for emerging network-related conditions, such as psychiatric disorders; and 3) Intelligent BMI SoCs for movement recovery following paralysis.
Enhancing Epileptic Seizure Detection with EEG Feature Embeddings
Oct 28, 2023



Abstract:Epilepsy is one of the most prevalent brain disorders that disrupts the lives of millions worldwide. For patients with drug-resistant seizures, there exist implantable devices capable of monitoring neural activity, promptly triggering neurostimulation to regulate seizures, or alerting patients of potential episodes. Next-generation seizure detection systems heavily rely on high-accuracy machine learning-based classifiers to detect the seizure onset. Here, we propose to enhance the seizure detection performance by learning informative embeddings of the EEG signal. We empirically demonstrate, for the first time, that converting raw EEG signals to appropriate embeddings can significantly boost the performance of seizure detection algorithms. Importantly, we show that embedding features, which converts the raw EEG into an alternative representation, is beneficial for various machine learning models such as Logistic Regression, Multi-Layer Perceptron, Support Vector Machines, and Gradient Boosted Trees. The experiments were conducted on the CHB-MIT scalp EEG dataset. With the proposed EEG feature embeddings, we achieve significant improvements in sensitivity, specificity, and AUC score across multiple models. By employing this approach alongside an SVM classifier, we were able to attain state-of-the-art classification performance with a sensitivity of 100% and specificity of 99%, setting a new benchmark in the field.
XTab: Cross-table Pretraining for Tabular Transformers
May 10, 2023



Abstract:The success of self-supervised learning in computer vision and natural language processing has motivated pretraining methods on tabular data. However, most existing tabular self-supervised learning models fail to leverage information across multiple data tables and cannot generalize to new tables. In this work, we introduce XTab, a framework for cross-table pretraining of tabular transformers on datasets from various domains. We address the challenge of inconsistent column types and quantities among tables by utilizing independent featurizers and using federated learning to pretrain the shared component. Tested on 84 tabular prediction tasks from the OpenML-AutoML Benchmark (AMLB), we show that (1) XTab consistently boosts the generalizability, learning speed, and performance of multiple tabular transformers, (2) by pretraining FT-Transformer via XTab, we achieve superior performance than other state-of-the-art tabular deep learning models on various tasks such as regression, binary, and multiclass classification.
An Accurate and Hardware-Efficient Dual Spike Detector for Implantable Neural Interfaces
Aug 29, 2022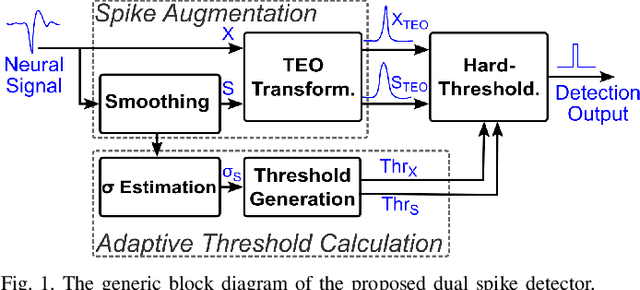
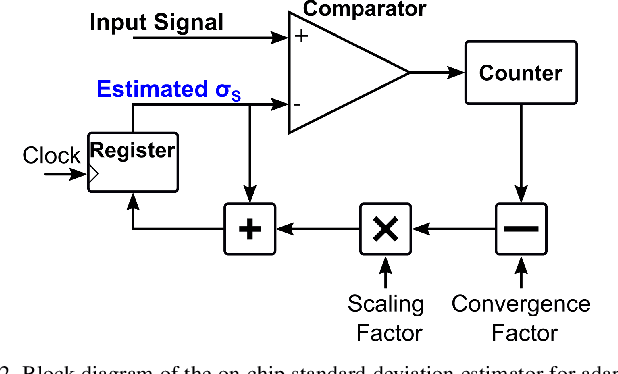
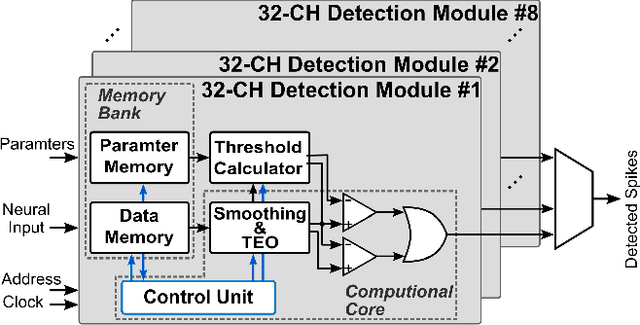
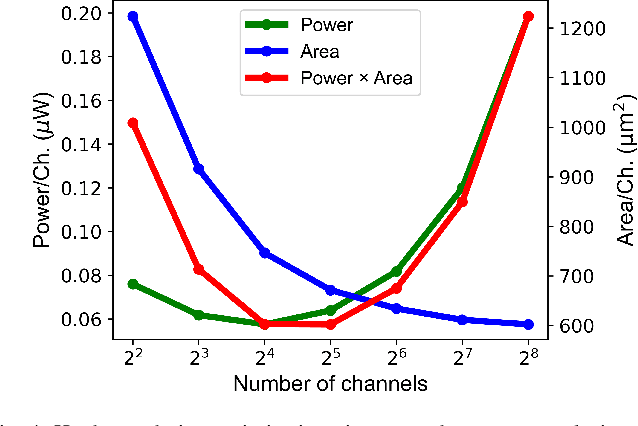
Abstract:Spike detection plays a central role in neural data processing and brain-machine interfaces (BMIs). A challenge for future-generation implantable BMIs is to build a spike detector that features both low hardware cost and high performance. In this work, we propose a novel hardware-efficient and high-performance spike detector for implantable BMIs. The proposed design is based on a dual-detector architecture with adaptive threshold estimation. The dual-detector comprises two separate TEO-based detectors that distinguish a spike occurrence based on its discriminating features in both high and low noise scenarios. We evaluated the proposed spike detection algorithm on the Wave Clus dataset. It achieved an average detection accuracy of 98.9%, and over 95% in high-noise scenarios, ensuring the reliability of our method. When realized in hardware with a sampling rate of 16kHz and 7-bits resolution, the detection accuracy is 97.4%. Designed in 65nm TSMC process, a 256-channel detector based on this architecture occupies only 682$\mu m^2$ /Channel and consumes 0.07$\mu$W/Channel, improving over the state-of-the-art spike detectors by 39.7% in power consumption and 78.8% in area, while maintaining a high accuracy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge