Uisub Shin
Intelligent Neural Interfaces: An Emerging Era in Neurotechnology
May 13, 2024Abstract:Integrating smart algorithms on neural devices presents significant opportunities for various brain disorders. In this paper, we review the latest advancements in the development of three categories of intelligent neural prostheses featuring embedded signal processing on the implantable or wearable device. These include: 1) Neural interfaces for closed-loop symptom tracking and responsive stimulation; 2) Neural interfaces for emerging network-related conditions, such as psychiatric disorders; and 3) Intelligent BMI SoCs for movement recovery following paralysis.
A 16-Channel Low-Power Neural Connectivity Extraction and Phase-Locked Deep Brain Stimulation SoC
Jul 03, 2022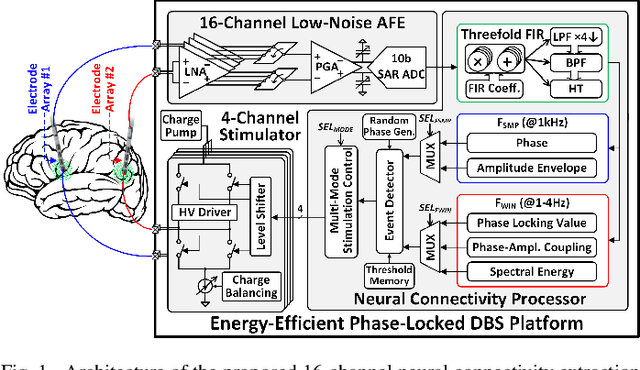
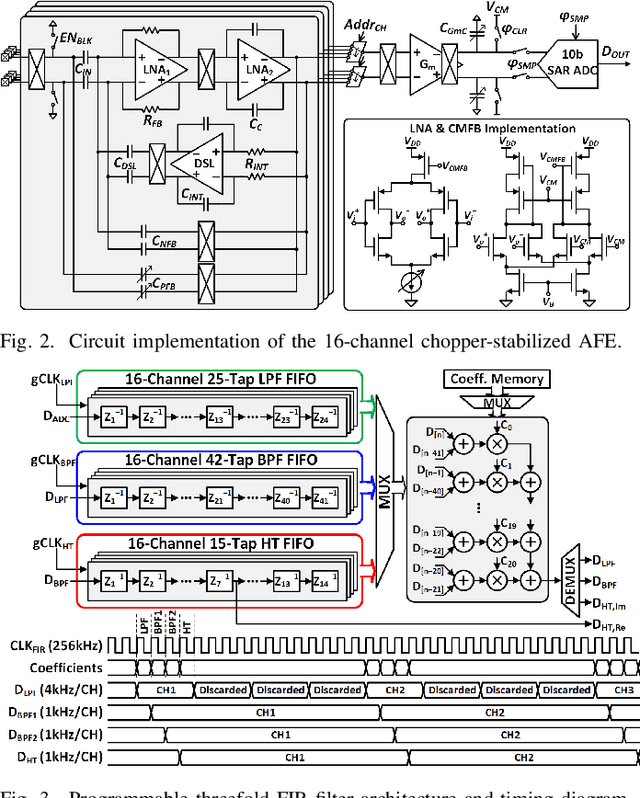
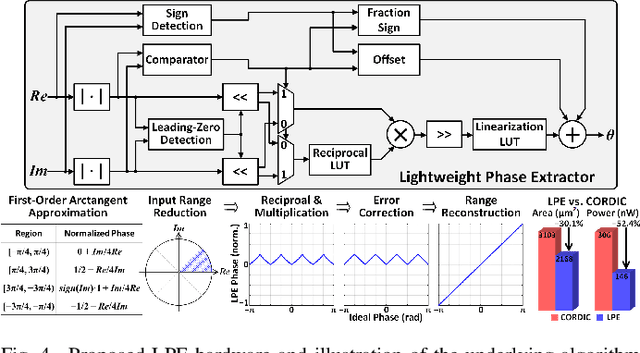
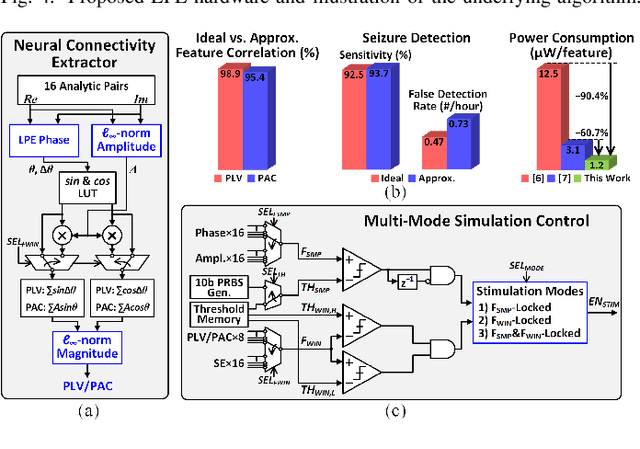
Abstract:Growing evidence suggests that phase-locked deep brain stimulation (DBS) can effectively regulate abnormal brain connectivity in neurological and psychiatric disorders. This letter therefore presents a low-power SoC with both neural connectivity extraction and phase-locked DBS capabilities. A 16-channel low-noise analog front-end (AFE) records local field potentials (LFPs) from multiple brain regions with precise gain matching. A novel low-complexity phase estimator and neural connectivity processor subsequently enable energy-efficient, yet accurate measurement of the instantaneous phase and cross-regional synchrony measures. Through flexible combination of neural biomarkers such as phase synchrony and spectral energy, a four-channel charge-balanced neurostimulator is triggered to treat various pathological brain conditions. Fabricated in 65nm CMOS, the SoC occupies a silicon area of 2.24mm2 and consumes 60uW, achieving over 60% power saving in neural connectivity extraction compared to the state-of-the-art. Extensive in-vivo measurements demonstrate multi-channel LFP recording, real-time extraction of phase and neural connectivity measures, and phase-locked stimulation in rats.
NeuralTree: A 256-Channel 0.227uJ/class Versatile Neural Activity Classification and Closed-Loop Neuromodulation SoC
May 21, 2022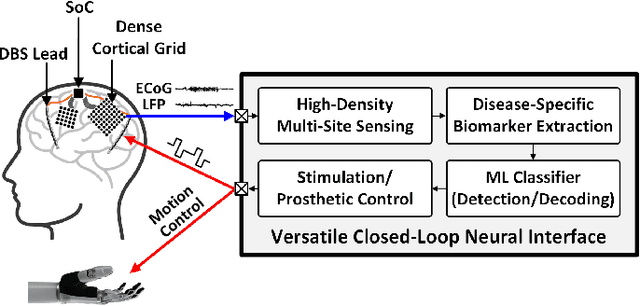
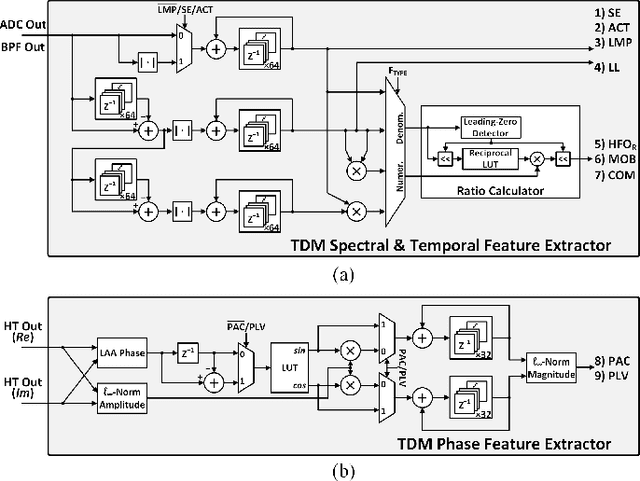
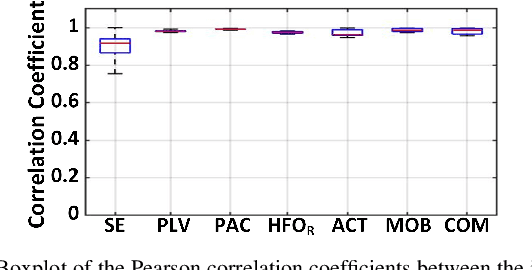
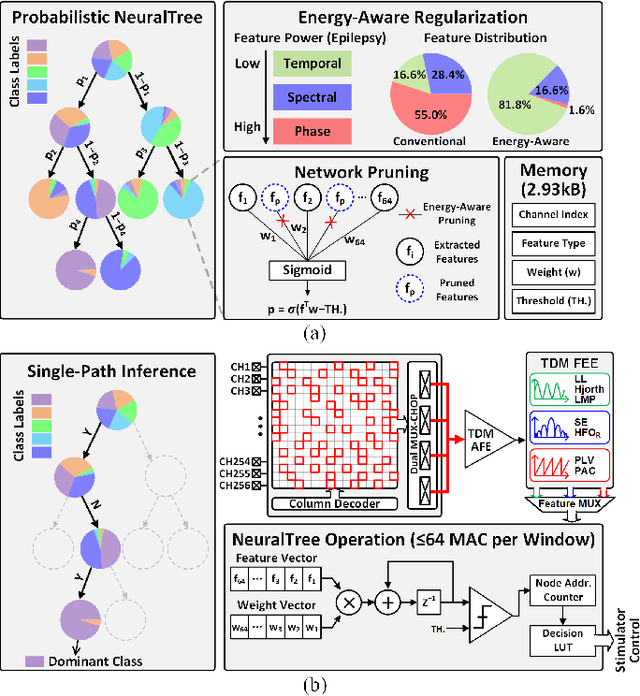
Abstract:Closed-loop neural interfaces with on-chip machine learning can detect and suppress disease symptoms in neurological disorders or restore lost functions in paralyzed patients. While high-density neural recording can provide rich neural activity information for accurate disease-state detection, existing systems have low channel count and poor scalability, which could limit their therapeutic efficacy. This work presents a highly scalable and versatile closed-loop neural interface SoC that can overcome these limitations. A 256-channel time-division multiplexed (TDM) front-end with a two-step fast-settling mixed-signal DC servo loop (DSL) is proposed to record high-spatial-resolution neural activity and perform channel-selective brain-state inference. A tree-structured neural network (NeuralTree) classification processor extracts a rich set of neural biomarkers in a patient- and disease-specific manner. Trained with an energy-aware learning algorithm, the NeuralTree classifier detects the symptoms of underlying disorders (e.g., epilepsy and movement disorders) at an optimal energy-accuracy trade-off. A 16-channel high-voltage (HV) compliant neurostimulator closes the therapeutic loop by delivering charge-balanced biphasic current pulses to the brain. The proposed SoC was fabricated in 65nm CMOS and achieved a 0.227uJ/class energy efficiency in a compact area of 0.014mm^2/channel. The SoC was extensively verified on human electroencephalography (EEG) and intracranial EEG (iEEG) epilepsy datasets, obtaining 95.6%/94% sensitivity and 96.8%/96.9% specificity, respectively. In-vivo neural recordings using soft uECoG arrays and multi-domain biomarker extraction were further performed on a rat model of epilepsy. In addition, for the first time in literature, on-chip classification of rest-state tremor in Parkinson's disease from human local field potentials (LFPs) was demonstrated.
Closed-Loop Neural Prostheses with On-Chip Intelligence: A Review and A Low-Latency Machine Learning Model for Brain State Detection
Sep 13, 2021

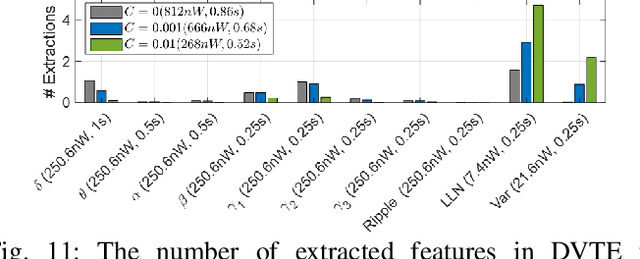
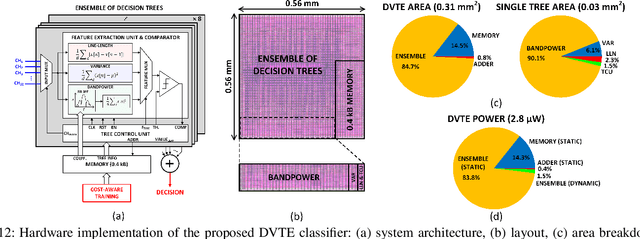
Abstract:The application of closed-loop approaches in systems neuroscience and therapeutic stimulation holds great promise for revolutionizing our understanding of the brain and for developing novel neuromodulation therapies to restore lost functions. Neural prostheses capable of multi-channel neural recording, on-site signal processing, rapid symptom detection, and closed-loop stimulation are critical to enabling such novel treatments. However, the existing closed-loop neuromodulation devices are too simplistic and lack sufficient on-chip processing and intelligence. In this paper, we first discuss both commercial and investigational closed-loop neuromodulation devices for brain disorders. Next, we review state-of-the-art neural prostheses with on-chip machine learning, focusing on application-specific integrated circuits (ASIC). System requirements, performance and hardware comparisons, design trade-offs, and hardware optimization techniques are discussed. To facilitate a fair comparison and guide design choices among various on-chip classifiers, we propose a new energy-area (E-A) efficiency figure of merit that evaluates hardware efficiency and multi-channel scalability. Finally, we present several techniques to improve the key design metrics of tree-based on-chip classifiers, both in the context of ensemble methods and oblique structures.
Closed-Loop Neural Interfaces with Embedded Machine Learning
Oct 21, 2020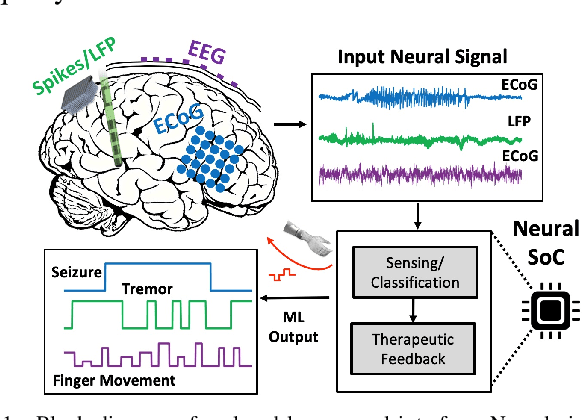
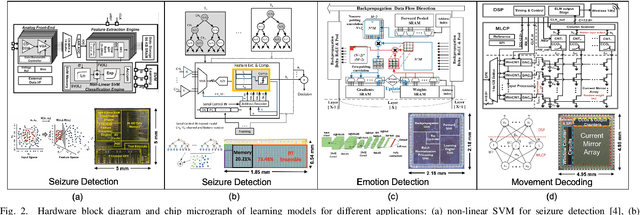
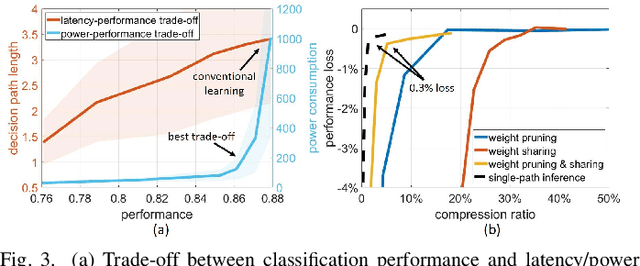
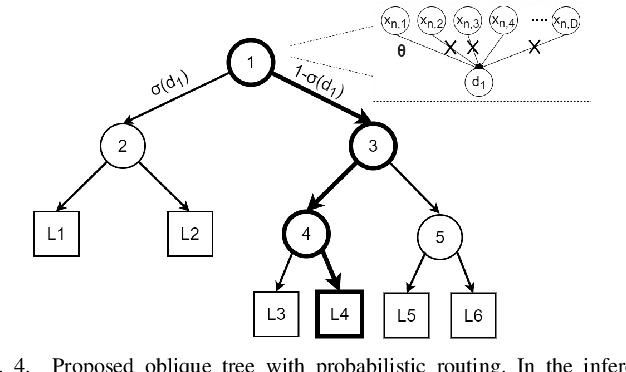
Abstract:Neural interfaces capable of multi-site electrical recording, on-site signal classification, and closed-loop therapy are critical for the diagnosis and treatment of neurological disorders. However, deploying machine learning algorithms on low-power neural devices is challenging, given the tight constraints on computational and memory resources for such devices. In this paper, we review the recent developments in embedding machine learning in neural interfaces, with a focus on design trade-offs and hardware efficiency. We also present our optimized tree-based model for low-power and memory-efficient classification of neural signal in brain implants. Using energy-aware learning and model compression, we show that the proposed oblique trees can outperform conventional machine learning models in applications such as seizure or tremor detection and motor decoding.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge