Laura Daza
No Data? No Problem: Robust Vision-Tabular Learning with Missing Values
Dec 22, 2025



Abstract:Large-scale medical biobanks provide imaging data complemented by extensive tabular information, such as demographics or clinical measurements. However, this abundance of tabular attributes does not reflect real-world datasets, where only a subset of attributes may be available. This discrepancy calls for methods that can leverage all the tabular data during training while remaining robust to missing values at inference. To address this challenge, we propose RoVTL (Robust Vision-Tabular Learning), a framework designed to handle any level of tabular data availability, from 0% to 100%. RoVTL comprises two key stages: contrastive pretraining, where we introduce tabular attribute missingness as data augmentation to promote robustness, and downstream task tuning using a gated cross-attention module for multimodal fusion. During fine-tuning, we employ a novel Tabular More vs. Fewer loss that ranks performance based on the amount of available tabular data. Combined with disentangled gradient learning, this enables consistent performance across all tabular data completeness scenarios. We evaluate RoVTL on cardiac MRI scans from the UK Biobank, demonstrating superior robustness to missing tabular data compared to prior methods. Furthermore, RoVTL successfully generalizes to an external cardiac MRI dataset for multimodal disease classification, and extends to the natural images domain, achieving robust performance on a car advertisements dataset. The code is available at https://github.com/marteczkah/RoVTL.
Video Swin Transformers for Egocentric Video Understanding @ Ego4D Challenges 2022
Jul 22, 2022
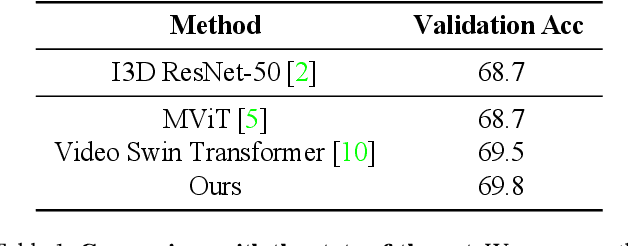
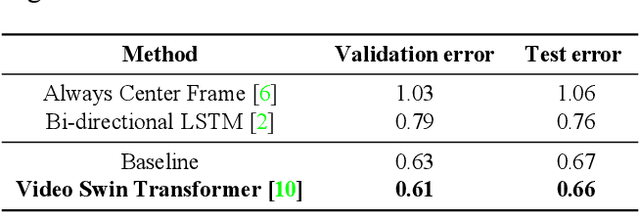
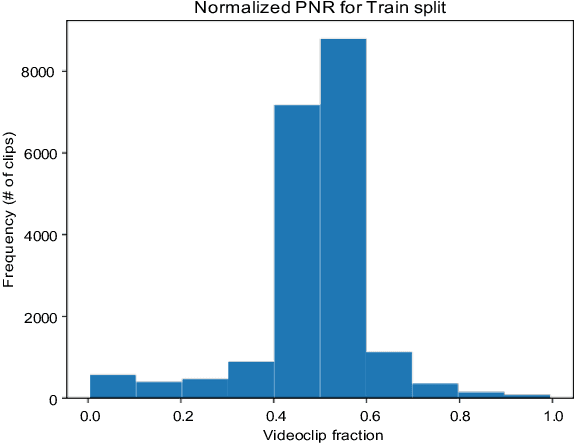
Abstract:We implemented Video Swin Transformer as a base architecture for the tasks of Point-of-No-Return temporal localization and Object State Change Classification. Our method achieved competitive performance on both challenges.
QU-BraTS: MICCAI BraTS 2020 Challenge on Quantifying Uncertainty in Brain Tumor Segmentation -- Analysis of Ranking Metrics and Benchmarking Results
Dec 19, 2021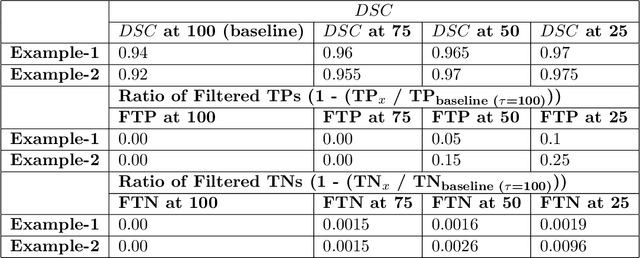
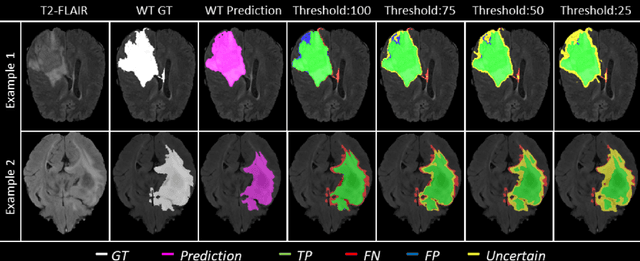
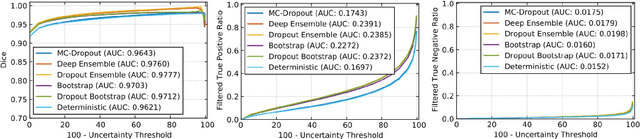
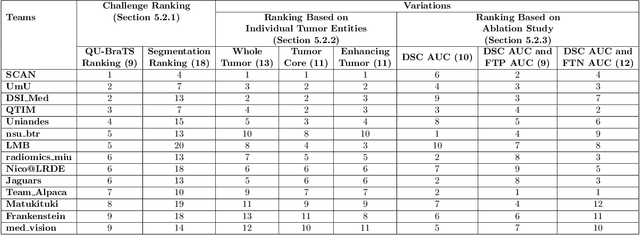
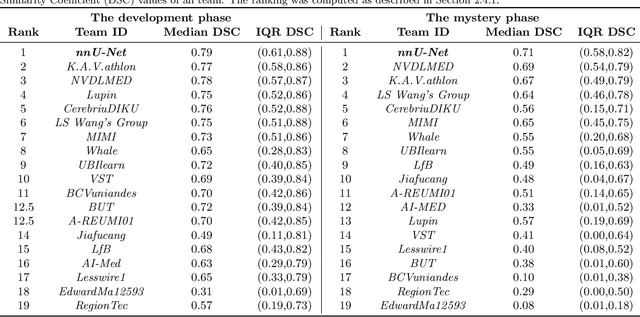
Abstract:Deep learning (DL) models have provided the state-of-the-art performance in a wide variety of medical imaging benchmarking challenges, including the Brain Tumor Segmentation (BraTS) challenges. However, the task of focal pathology multi-compartment segmentation (e.g., tumor and lesion sub-regions) is particularly challenging, and potential errors hinder the translation of DL models into clinical workflows. Quantifying the reliability of DL model predictions in the form of uncertainties, could enable clinical review of the most uncertain regions, thereby building trust and paving the way towards clinical translation. Recently, a number of uncertainty estimation methods have been introduced for DL medical image segmentation tasks. Developing metrics to evaluate and compare the performance of uncertainty measures will assist the end-user in making more informed decisions. In this study, we explore and evaluate a metric developed during the BraTS 2019-2020 task on uncertainty quantification (QU-BraTS), and designed to assess and rank uncertainty estimates for brain tumor multi-compartment segmentation. This metric (1) rewards uncertainty estimates that produce high confidence in correct assertions, and those that assign low confidence levels at incorrect assertions, and (2) penalizes uncertainty measures that lead to a higher percentages of under-confident correct assertions. We further benchmark the segmentation uncertainties generated by 14 independent participating teams of QU-BraTS 2020, all of which also participated in the main BraTS segmentation task. Overall, our findings confirm the importance and complementary value that uncertainty estimates provide to segmentation algorithms, and hence highlight the need for uncertainty quantification in medical image analyses. Our evaluation code is made publicly available at https://github.com/RagMeh11/QU-BraTS.
Towards Robust General Medical Image Segmentation
Jul 09, 2021



Abstract:The reliability of Deep Learning systems depends on their accuracy but also on their robustness against adversarial perturbations to the input data. Several attacks and defenses have been proposed to improve the performance of Deep Neural Networks under the presence of adversarial noise in the natural image domain. However, robustness in computer-aided diagnosis for volumetric data has only been explored for specific tasks and with limited attacks. We propose a new framework to assess the robustness of general medical image segmentation systems. Our contributions are two-fold: (i) we propose a new benchmark to evaluate robustness in the context of the Medical Segmentation Decathlon (MSD) by extending the recent AutoAttack natural image classification framework to the domain of volumetric data segmentation, and (ii) we present a novel lattice architecture for RObust Generic medical image segmentation (ROG). Our results show that ROG is capable of generalizing across different tasks of the MSD and largely surpasses the state-of-the-art under sophisticated adversarial attacks.
The Medical Segmentation Decathlon
Jun 10, 2021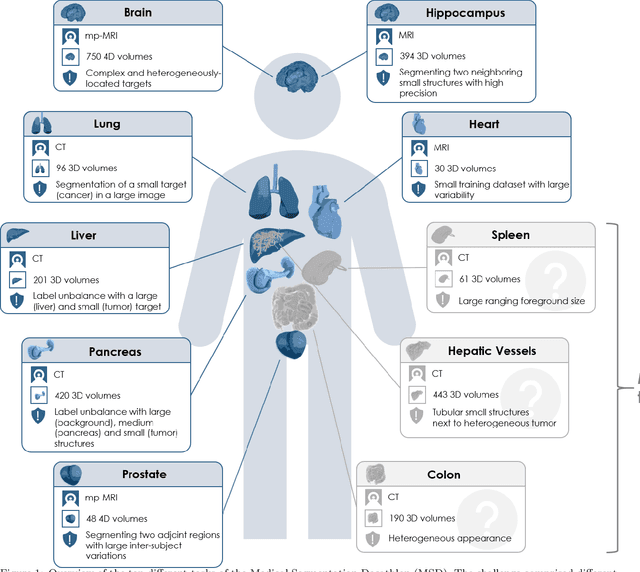
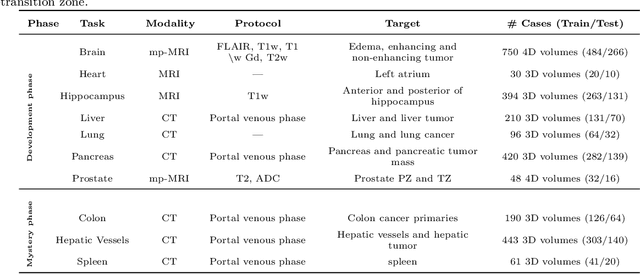
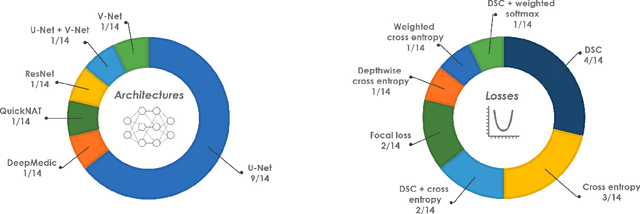
Abstract:International challenges have become the de facto standard for comparative assessment of image analysis algorithms given a specific task. Segmentation is so far the most widely investigated medical image processing task, but the various segmentation challenges have typically been organized in isolation, such that algorithm development was driven by the need to tackle a single specific clinical problem. We hypothesized that a method capable of performing well on multiple tasks will generalize well to a previously unseen task and potentially outperform a custom-designed solution. To investigate the hypothesis, we organized the Medical Segmentation Decathlon (MSD) - a biomedical image analysis challenge, in which algorithms compete in a multitude of both tasks and modalities. The underlying data set was designed to explore the axis of difficulties typically encountered when dealing with medical images, such as small data sets, unbalanced labels, multi-site data and small objects. The MSD challenge confirmed that algorithms with a consistent good performance on a set of tasks preserved their good average performance on a different set of previously unseen tasks. Moreover, by monitoring the MSD winner for two years, we found that this algorithm continued generalizing well to a wide range of other clinical problems, further confirming our hypothesis. Three main conclusions can be drawn from this study: (1) state-of-the-art image segmentation algorithms are mature, accurate, and generalize well when retrained on unseen tasks; (2) consistent algorithmic performance across multiple tasks is a strong surrogate of algorithmic generalizability; (3) the training of accurate AI segmentation models is now commoditized to non AI experts.
SIMBA: Specific Identity Markers for Bone Age Assessment
Jul 13, 2020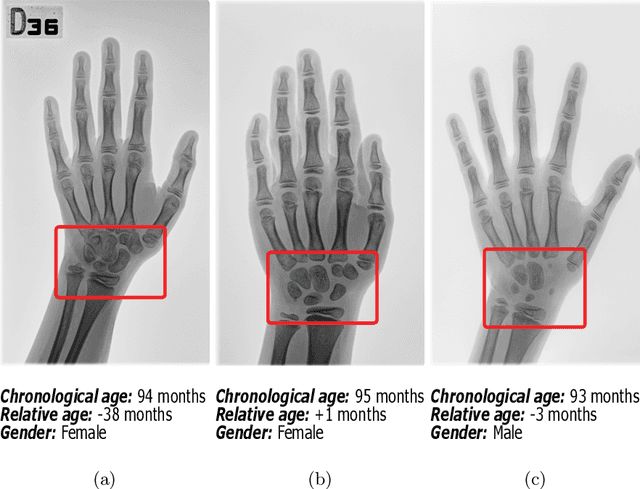
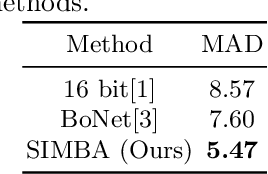
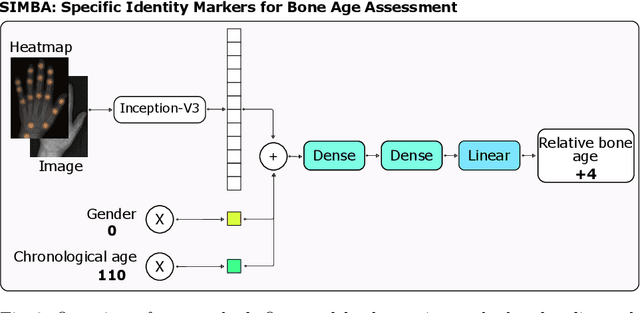
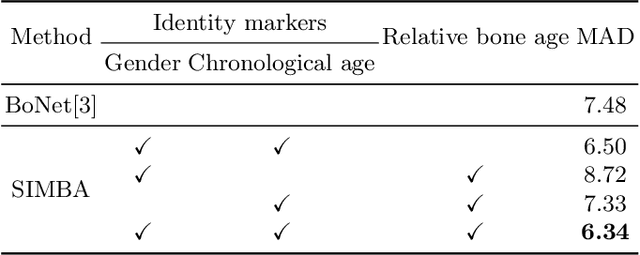
Abstract:Bone Age Assessment (BAA) is a task performed by radiologists to diagnose abnormal growth in a child. In manual approaches, radiologists take into account different identity markers when calculating bone age, i.e., chronological age and gender. However, the current automated Bone Age Assessment methods do not completely exploit the information present in the patient's metadata. With this lack of available methods as motivation, we present SIMBA: Specific Identity Markers for Bone Age Assessment. SIMBA is a novel approach for the task of BAA based on the use of identity markers. For this purpose, we build upon the state-of-the-art model, fusing the information present in the identity markers with the visual features created from the original hand radiograph. We then use this robust representation to estimate the patient's relative bone age: the difference between chronological age and bone age. We validate SIMBA on the Radiological Hand Pose Estimation dataset and find that it outperforms previous state-of-the-art methods. SIMBA sets a trend of a new wave of Computer-aided Diagnosis methods that incorporate all of the data that is available regarding a patient. To promote further research in this area and ensure reproducibility we will provide the source code as well as the pre-trained models of SIMBA.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge