Kyunghyun Paeng
OCELOT 2023: Cell Detection from Cell-Tissue Interaction Challenge
Sep 11, 2025Abstract:Pathologists routinely alternate between different magnifications when examining Whole-Slide Images, allowing them to evaluate both broad tissue morphology and intricate cellular details to form comprehensive diagnoses. However, existing deep learning-based cell detection models struggle to replicate these behaviors and learn the interdependent semantics between structures at different magnifications. A key barrier in the field is the lack of datasets with multi-scale overlapping cell and tissue annotations. The OCELOT 2023 challenge was initiated to gather insights from the community to validate the hypothesis that understanding cell and tissue (cell-tissue) interactions is crucial for achieving human-level performance, and to accelerate the research in this field. The challenge dataset includes overlapping cell detection and tissue segmentation annotations from six organs, comprising 673 pairs sourced from 306 The Cancer Genome Atlas (TCGA) Whole-Slide Images with hematoxylin and eosin staining, divided into training, validation, and test subsets. Participants presented models that significantly enhanced the understanding of cell-tissue relationships. Top entries achieved up to a 7.99 increase in F1-score on the test set compared to the baseline cell-only model that did not incorporate cell-tissue relationships. This is a substantial improvement in performance over traditional cell-only detection methods, demonstrating the need for incorporating multi-scale semantics into the models. This paper provides a comparative analysis of the methods used by participants, highlighting innovative strategies implemented in the OCELOT 2023 challenge.
* This is the accepted manuscript of an article published in Medical Image Analysis (Elsevier). The final version is available at: https://doi.org/10.1016/j.media.2025.103751
SCORPION: Addressing Scanner-Induced Variability in Histopathology
Jul 28, 2025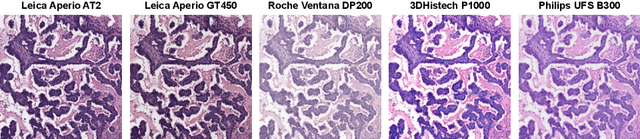
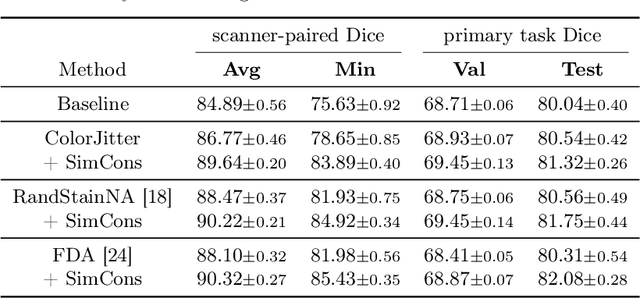


Abstract:Ensuring reliable model performance across diverse domains is a critical challenge in computational pathology. A particular source of variability in Whole-Slide Images is introduced by differences in digital scanners, thus calling for better scanner generalization. This is critical for the real-world adoption of computational pathology, where the scanning devices may differ per institution or hospital, and the model should not be dependent on scanner-induced details, which can ultimately affect the patient's diagnosis and treatment planning. However, past efforts have primarily focused on standard domain generalization settings, evaluating on unseen scanners during training, without directly evaluating consistency across scanners for the same tissue. To overcome this limitation, we introduce SCORPION, a new dataset explicitly designed to evaluate model reliability under scanner variability. SCORPION includes 480 tissue samples, each scanned with 5 scanners, yielding 2,400 spatially aligned patches. This scanner-paired design allows for the isolation of scanner-induced variability, enabling a rigorous evaluation of model consistency while controlling for differences in tissue composition. Furthermore, we propose SimCons, a flexible framework that combines augmentation-based domain generalization techniques with a consistency loss to explicitly address scanner generalization. We empirically show that SimCons improves model consistency on varying scanners without compromising task-specific performance. By releasing the SCORPION dataset and proposing SimCons, we provide the research community with a crucial resource for evaluating and improving model consistency across diverse scanners, setting a new standard for reliability testing.
Generalizing AI-driven Assessment of Immunohistochemistry across Immunostains and Cancer Types: A Universal Immunohistochemistry Analyzer
Jul 30, 2024Abstract:Despite advancements in methodologies, immunohistochemistry (IHC) remains the most utilized ancillary test for histopathologic and companion diagnostics in targeted therapies. However, objective IHC assessment poses challenges. Artificial intelligence (AI) has emerged as a potential solution, yet its development requires extensive training for each cancer and IHC type, limiting versatility. We developed a Universal IHC (UIHC) analyzer, an AI model for interpreting IHC images regardless of tumor or IHC types, using training datasets from various cancers stained for PD-L1 and/or HER2. This multi-cohort trained model outperforms conventional single-cohort models in interpreting unseen IHCs (Kappa score 0.578 vs. up to 0.509) and consistently shows superior performance across different positive staining cutoff values. Qualitative analysis reveals that UIHC effectively clusters patches based on expression levels. The UIHC model also quantitatively assesses c-MET expression with MET mutations, representing a significant advancement in AI application in the era of personalized medicine and accumulating novel biomarkers.
OCELOT: Overlapped Cell on Tissue Dataset for Histopathology
Mar 24, 2023



Abstract:Cell detection is a fundamental task in computational pathology that can be used for extracting high-level medical information from whole-slide images. For accurate cell detection, pathologists often zoom out to understand the tissue-level structures and zoom in to classify cells based on their morphology and the surrounding context. However, there is a lack of efforts to reflect such behaviors by pathologists in the cell detection models, mainly due to the lack of datasets containing both cell and tissue annotations with overlapping regions. To overcome this limitation, we propose and publicly release OCELOT, a dataset purposely dedicated to the study of cell-tissue relationships for cell detection in histopathology. OCELOT provides overlapping cell and tissue annotations on images acquired from multiple organs. Within this setting, we also propose multi-task learning approaches that benefit from learning both cell and tissue tasks simultaneously. When compared against a model trained only for the cell detection task, our proposed approaches improve cell detection performance on 3 datasets: proposed OCELOT, public TIGER, and internal CARP datasets. On the OCELOT test set in particular, we show up to 6.79 improvement in F1-score. We believe the contributions of this paper, including the release of the OCELOT dataset at https://lunit-io.github.io/research/publications/ocelot are a crucial starting point toward the important research direction of incorporating cell-tissue relationships in computation pathology.
PseudoEdgeNet: Nuclei Segmentation only with Point Annotations
Jul 22, 2019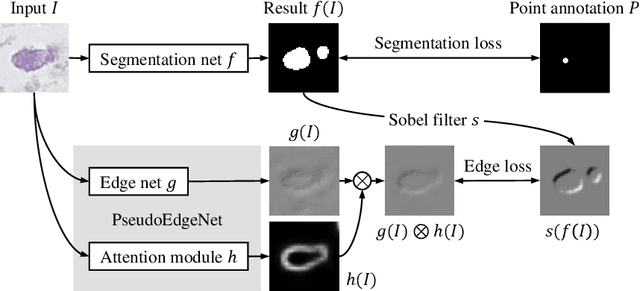
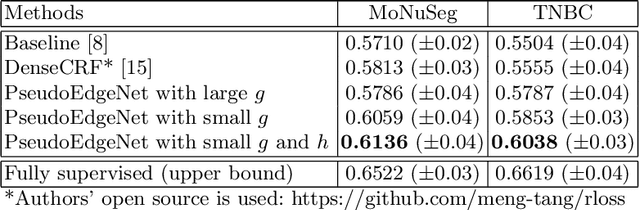
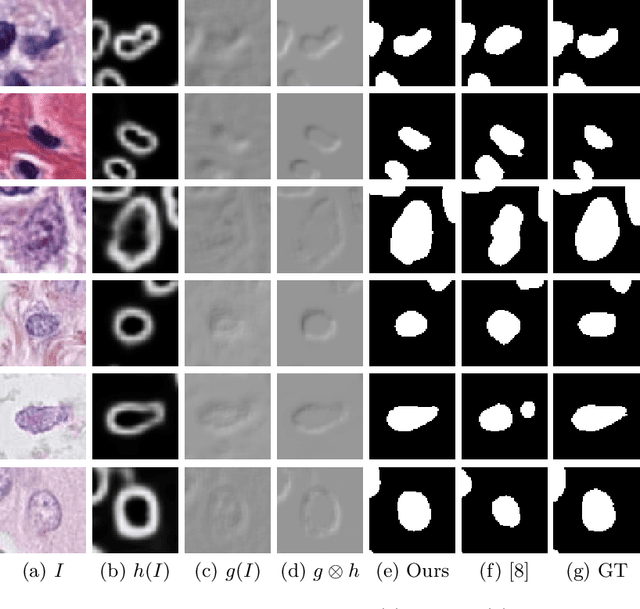
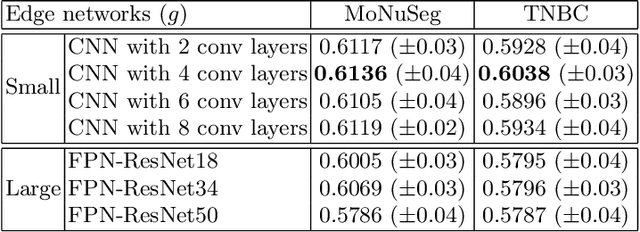
Abstract:Nuclei segmentation is one of the important tasks for whole slide image analysis in digital pathology. With the drastic advance of deep learning, recent deep networks have demonstrated successful performance of the nuclei segmentation task. However, a major bottleneck to achieving good performance is the cost for annotation. A large network requires a large number of segmentation masks, and this annotation task is given to pathologists, not the public. In this paper, we propose a weakly supervised nuclei segmentation method, which requires only point annotations for training. This method can scale to large training set as marking a point of a nucleus is much cheaper than the fine segmentation mask. To this end, we introduce a novel auxiliary network, called PseudoEdgeNet, which guides the segmentation network to recognize nuclei edges even without edge annotations. We evaluate our method with two public datasets, and the results demonstrate that the method consistently outperforms other weakly supervised methods.
Predicting breast tumor proliferation from whole-slide images: the TUPAC16 challenge
Jul 22, 2018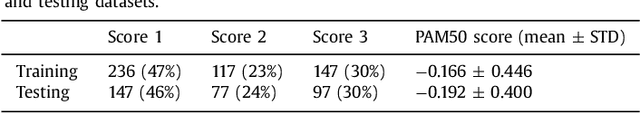
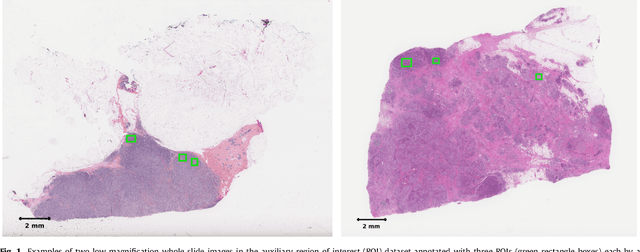
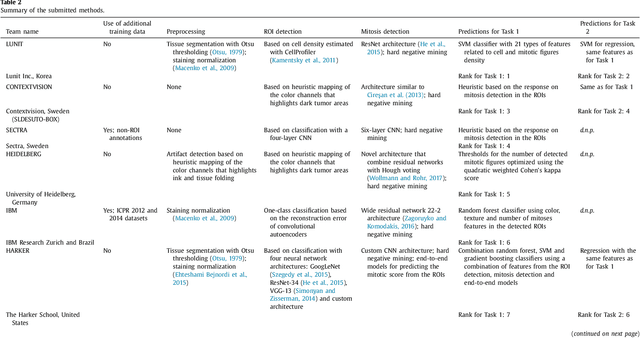
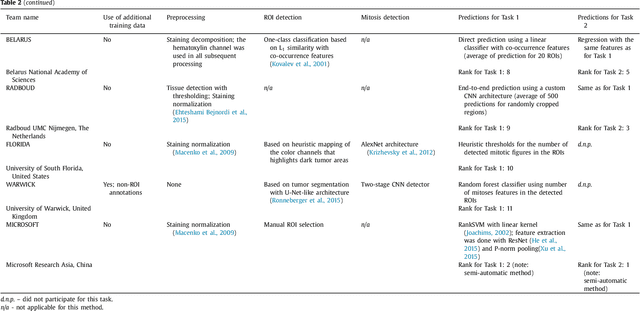
Abstract:Tumor proliferation is an important biomarker indicative of the prognosis of breast cancer patients. Assessment of tumor proliferation in a clinical setting is highly subjective and labor-intensive task. Previous efforts to automate tumor proliferation assessment by image analysis only focused on mitosis detection in predefined tumor regions. However, in a real-world scenario, automatic mitosis detection should be performed in whole-slide images (WSIs) and an automatic method should be able to produce a tumor proliferation score given a WSI as input. To address this, we organized the TUmor Proliferation Assessment Challenge 2016 (TUPAC16) on prediction of tumor proliferation scores from WSIs. The challenge dataset consisted of 500 training and 321 testing breast cancer histopathology WSIs. In order to ensure fair and independent evaluation, only the ground truth for the training dataset was provided to the challenge participants. The first task of the challenge was to predict mitotic scores, i.e., to reproduce the manual method of assessing tumor proliferation by a pathologist. The second task was to predict the gene expression based PAM50 proliferation scores from the WSI. The best performing automatic method for the first task achieved a quadratic-weighted Cohen's kappa score of $\kappa$ = 0.567, 95% CI [0.464, 0.671] between the predicted scores and the ground truth. For the second task, the predictions of the top method had a Spearman's correlation coefficient of r = 0.617, 95% CI [0.581 0.651] with the ground truth. This was the first study that investigated tumor proliferation assessment from WSIs. The achieved results are promising given the difficulty of the tasks and weakly-labelled nature of the ground truth. However, further research is needed to improve the practical utility of image analysis methods for this task.
A Robust and Effective Approach Towards Accurate Metastasis Detection and pN-stage Classification in Breast Cancer
May 30, 2018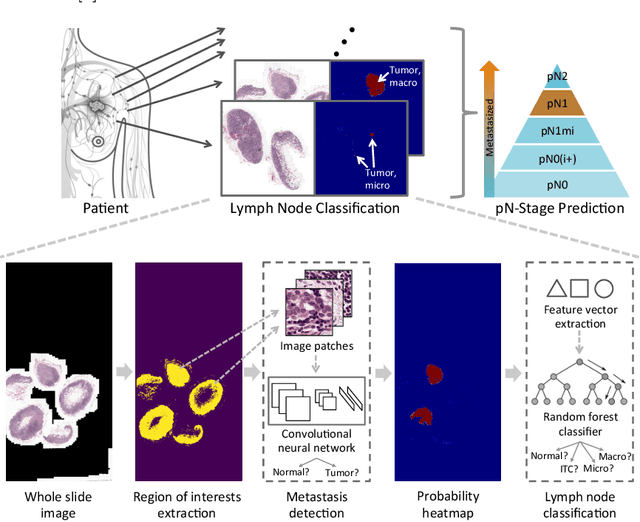
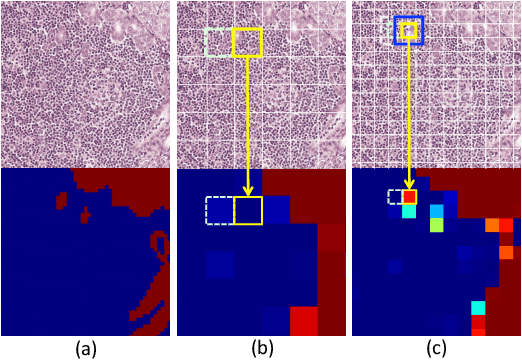
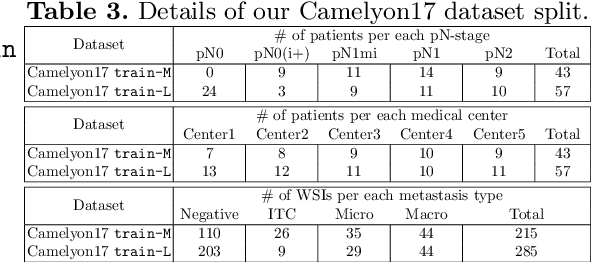
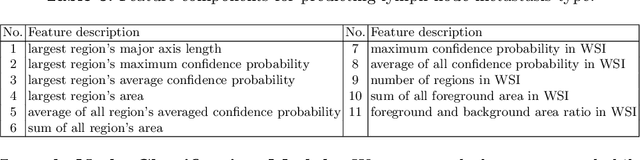
Abstract:Predicting TNM stage is the major determinant of breast cancer prognosis and treatment. The essential part of TNM stage classification is whether the cancer has metastasized to the regional lymph nodes (N-stage). Pathologic N-stage (pN-stage) is commonly performed by pathologists detecting metastasis in histological slides. However, this diagnostic procedure is prone to misinterpretation and would normally require extensive time by pathologists because of the sheer volume of data that needs a thorough review. Automated detection of lymph node metastasis and pN-stage prediction has a great potential to reduce their workload and help the pathologist. Recent advances in convolutional neural networks (CNN) have shown significant improvements in histological slide analysis, but accuracy is not optimized because of the difficulty in the handling of gigapixel images. In this paper, we propose a robust method for metastasis detection and pN-stage classification in breast cancer from multiple gigapixel pathology images in an effective way. pN-stage is predicted by combining patch-level CNN based metastasis detector and slide-level lymph node classifier. The proposed framework achieves a state-of-the-art quadratic weighted kappa score of 0.9203 on the Camelyon17 dataset, outperforming the previous winning method of the Camelyon17 challenge.
A Unified Framework for Tumor Proliferation Score Prediction in Breast Histopathology
Aug 11, 2017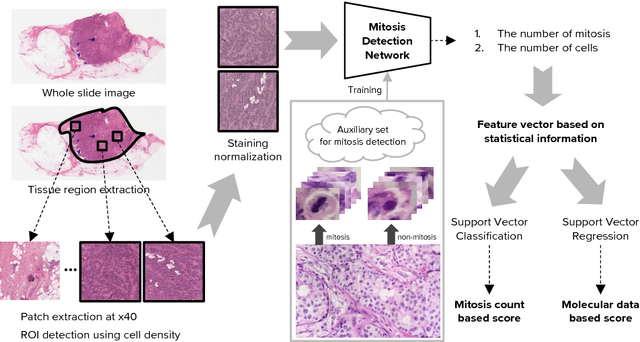
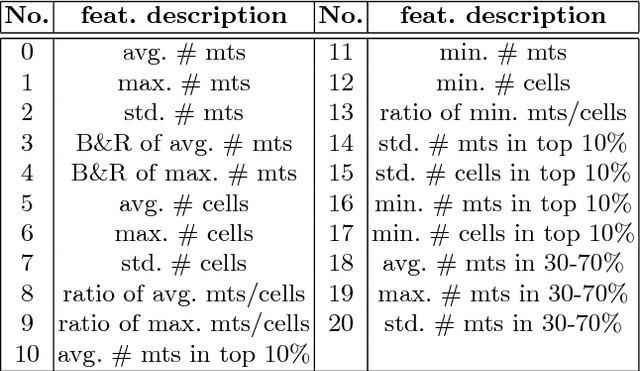
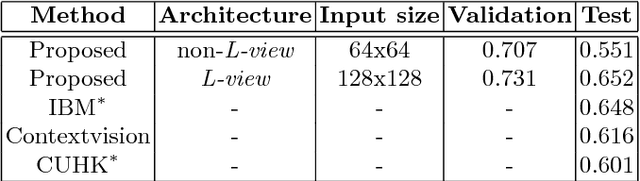
Abstract:We present a unified framework to predict tumor proliferation scores from breast histopathology whole slide images. Our system offers a fully automated solution to predicting both a molecular data-based, and a mitosis counting-based tumor proliferation score. The framework integrates three modules, each fine-tuned to maximize the overall performance: An image processing component for handling whole slide images, a deep learning based mitosis detection network, and a proliferation scores prediction module. We have achieved 0.567 quadratic weighted Cohen's kappa in mitosis counting-based score prediction and 0.652 F1-score in mitosis detection. On Spearman's correlation coefficient, which evaluates predictive accuracy on the molecular data based score, the system obtained 0.6171. Our approach won first place in all of the three tasks in Tumor Proliferation Assessment Challenge 2016 which is MICCAI grand challenge.
Action-Driven Object Detection with Top-Down Visual Attentions
Dec 20, 2016
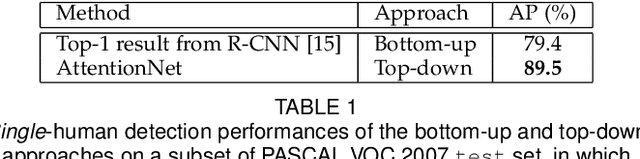
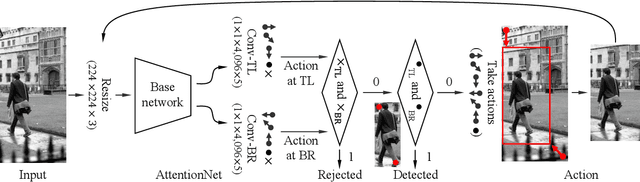
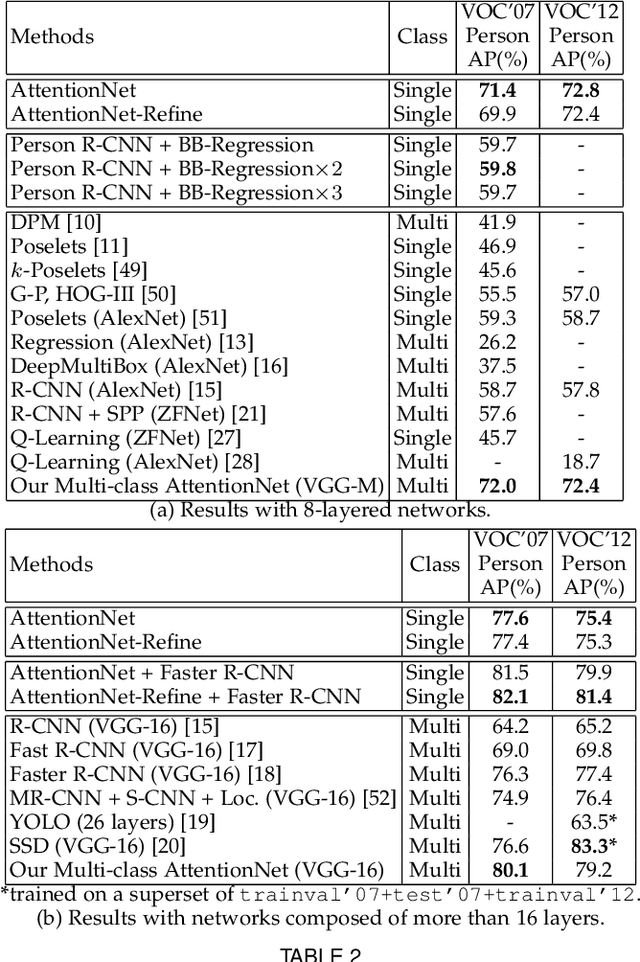
Abstract:A dominant paradigm for deep learning based object detection relies on a "bottom-up" approach using "passive" scoring of class agnostic proposals. These approaches are efficient but lack of holistic analysis of scene-level context. In this paper, we present an "action-driven" detection mechanism using our "top-down" visual attention model. We localize an object by taking sequential actions that the attention model provides. The attention model conditioned with an image region provides required actions to get closer toward a target object. An action at each time step is weak itself but an ensemble of the sequential actions makes a bounding-box accurately converge to a target object boundary. This attention model we call AttentionNet is composed of a convolutional neural network. During our whole detection procedure, we only utilize the actions from a single AttentionNet without any modules for object proposals nor post bounding-box regression. We evaluate our top-down detection mechanism over the PASCAL VOC series and ILSVRC CLS-LOC dataset, and achieve state-of-the-art performances compared to the major bottom-up detection methods. In particular, our detection mechanism shows a strong advantage in elaborate localization by outperforming Faster R-CNN with a margin of +7.1% over PASCAL VOC 2007 when we increase the IoU threshold for positive detection to 0.7.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge