Hersh Chandarana
Hybrid Learning: A Novel Combination of Self-Supervised and Supervised Learning for MRI Reconstruction without High-Quality Training Reference
May 09, 2025Abstract:Purpose: Deep learning has demonstrated strong potential for MRI reconstruction, but conventional supervised learning methods require high-quality reference images, which are often unavailable in practice. Self-supervised learning offers an alternative, yet its performance degrades at high acceleration rates. To overcome these limitations, we propose hybrid learning, a novel two-stage training framework that combines self-supervised and supervised learning for robust image reconstruction. Methods: Hybrid learning is implemented in two sequential stages. In the first stage, self-supervised learning is employed to generate improved images from noisy or undersampled reference data. These enhanced images then serve as pseudo-ground truths for the second stage, which uses supervised learning to refine reconstruction performance and support higher acceleration rates. We evaluated hybrid learning in two representative applications: (1) accelerated 0.55T spiral-UTE lung MRI using noisy reference data, and (2) 3D T1 mapping of the brain without access to fully sampled ground truth. Results: For spiral-UTE lung MRI, hybrid learning consistently improved image quality over both self-supervised and conventional supervised methods across different acceleration rates, as measured by SSIM and NMSE. For 3D T1 mapping, hybrid learning achieved superior T1 quantification accuracy across a wide dynamic range, outperforming self-supervised learning in all tested conditions. Conclusions: Hybrid learning provides a practical and effective solution for training deep MRI reconstruction networks when only low-quality or incomplete reference data are available. It enables improved image quality and accurate quantitative mapping across different applications and field strengths, representing a promising technique toward broader clinical deployment of deep learning-based MRI.
Self-Supervised Noise Adaptive MRI Denoising via Repetition to Repetition (Rep2Rep) Learning
Apr 24, 2025Abstract:Purpose: This work proposes a novel self-supervised noise-adaptive image denoising framework, called Repetition to Repetition (Rep2Rep) learning, for low-field (<1T) MRI applications. Methods: Rep2Rep learning extends the Noise2Noise framework by training a neural network on two repeated MRI acquisitions, using one repetition as input and another as target, without requiring ground-truth data. It incorporates noise-adaptive training, enabling denoising generalization across varying noise levels and flexible inference with any number of repetitions. Performance was evaluated on both synthetic noisy brain MRI and 0.55T prostate MRI data, and compared against supervised learning and Monte Carlo Stein's Unbiased Risk Estimator (MC-SURE). Results: Rep2Rep learning outperforms MC-SURE on both synthetic and 0.55T MRI datasets. On synthetic brain data, it achieved denoising quality comparable to supervised learning and surpassed MC-SURE, particularly in preserving structural details and reducing residual noise. On the 0.55T prostate MRI dataset, a reader study showed radiologists preferred Rep2Rep-denoised 2-average images over 8-average noisy images. Rep2Rep demonstrated robustness to noise-level discrepancies between training and inference, supporting its practical implementation. Conclusion: Rep2Rep learning offers an effective self-supervised denoising for low-field MRI by leveraging routinely acquired multi-repetition data. Its noise-adaptivity enables generalization to different SNR regimes without clean reference images. This makes Rep2Rep learning a promising tool for improving image quality and scan efficiency in low-field MRI.
FastMRI Prostate: A Publicly Available, Biparametric MRI Dataset to Advance Machine Learning for Prostate Cancer Imaging
Apr 18, 2023Abstract:The fastMRI brain and knee dataset has enabled significant advances in exploring reconstruction methods for improving speed and image quality for Magnetic Resonance Imaging (MRI) via novel, clinically relevant reconstruction approaches. In this study, we describe the April 2023 expansion of the fastMRI dataset to include biparametric prostate MRI data acquired on a clinical population. The dataset consists of raw k-space and reconstructed images for T2-weighted and diffusion-weighted sequences along with slice-level labels that indicate the presence and grade of prostate cancer. As has been the case with fastMRI, increasing accessibility to raw prostate MRI data will further facilitate research in MR image reconstruction and evaluation with the larger goal of improving the utility of MRI for prostate cancer detection and evaluation. The dataset is available at https://fastmri.med.nyu.edu.
On the Feasibility of Machine Learning Augmented Magnetic Resonance for Point-of-Care Identification of Disease
Feb 02, 2023



Abstract:Early detection of many life-threatening diseases (e.g., prostate and breast cancer) within at-risk population can improve clinical outcomes and reduce cost of care. While numerous disease-specific "screening" tests that are closer to Point-of-Care (POC) are in use for this task, their low specificity results in unnecessary biopsies, leading to avoidable patient trauma and wasteful healthcare spending. On the other hand, despite the high accuracy of Magnetic Resonance (MR) imaging in disease diagnosis, it is not used as a POC disease identification tool because of poor accessibility. The root cause of poor accessibility of MR stems from the requirement to reconstruct high-fidelity images, as it necessitates a lengthy and complex process of acquiring large quantities of high-quality k-space measurements. In this study we explore the feasibility of an ML-augmented MR pipeline that directly infers the disease sidestepping the image reconstruction process. We hypothesise that the disease classification task can be solved using a very small tailored subset of k-space data, compared to image reconstruction. Towards that end, we propose a method that performs two tasks: 1) identifies a subset of the k-space that maximizes disease identification accuracy, and 2) infers the disease directly using the identified k-space subset, bypassing the image reconstruction step. We validate our hypothesis by measuring the performance of the proposed system across multiple diseases and anatomies. We show that comparable performance to image-based classifiers, trained on images reconstructed with full k-space data, can be achieved using small quantities of data: 8% of the data for detecting multiple abnormalities in prostate and brain scans, and 5% of the data for knee abnormalities. To better understand the proposed approach and instigate future research, we provide an extensive analysis and release code.
fastMRI: An Open Dataset and Benchmarks for Accelerated MRI
Nov 21, 2018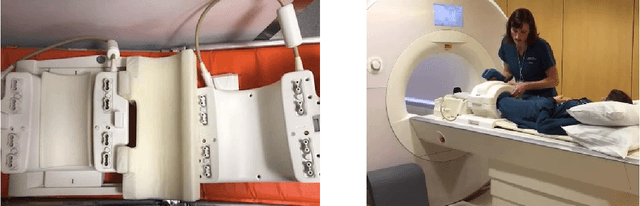
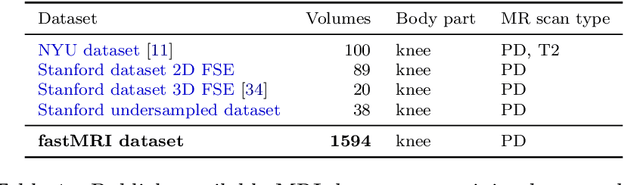
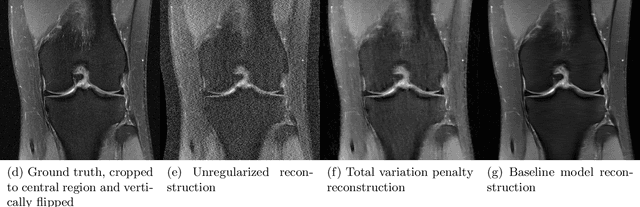
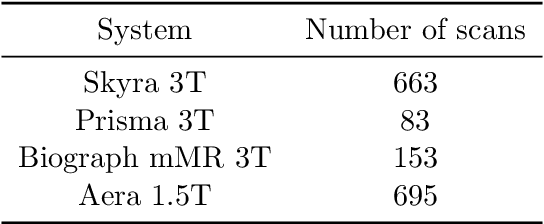
Abstract:Accelerating Magnetic Resonance Imaging (MRI) by taking fewer measurements has the potential to reduce medical costs, minimize stress to patients and make MRI possible in applications where it is currently prohibitively slow or expensive. We introduce the fastMRI dataset, a large-scale collection of both raw MR measurements and clinical MR images, that can be used for training and evaluation of machine-learning approaches to MR image reconstruction. By introducing standardized evaluation criteria and a freely-accessible dataset, our goal is to help the community make rapid advances in the state of the art for MR image reconstruction. We also provide a self-contained introduction to MRI for machine learning researchers with no medical imaging background.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge