Daniel Suter
Domain adaptation strategies for 3D reconstruction of the lumbar spine using real fluoroscopy data
Jan 29, 2024

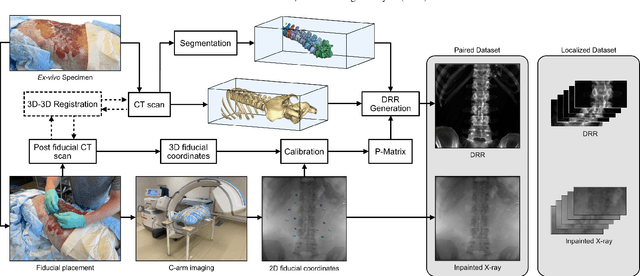

Abstract:This study tackles key obstacles in adopting surgical navigation in orthopedic surgeries, including time, cost, radiation, and workflow integration challenges. Recently, our work X23D showed an approach for generating 3D anatomical models of the spine from only a few intraoperative fluoroscopic images. This negates the need for conventional registration-based surgical navigation by creating a direct intraoperative 3D reconstruction of the anatomy. Despite these strides, the practical application of X23D has been limited by a domain gap between synthetic training data and real intraoperative images. In response, we devised a novel data collection protocol for a paired dataset consisting of synthetic and real fluoroscopic images from the same perspectives. Utilizing this dataset, we refined our deep learning model via transfer learning, effectively bridging the domain gap between synthetic and real X-ray data. A novel style transfer mechanism also allows us to convert real X-rays to mirror the synthetic domain, enabling our in-silico-trained X23D model to achieve high accuracy in real-world settings. Our results demonstrated that the refined model can rapidly generate accurate 3D reconstructions of the entire lumbar spine from as few as three intraoperative fluoroscopic shots. It achieved an 84% F1 score, matching the accuracy of our previous synthetic data-based research. Additionally, with a computational time of only 81.1 ms, our approach provides real-time capabilities essential for surgery integration. Through examining ideal imaging setups and view angle dependencies, we've further confirmed our system's practicality and dependability in clinical settings. Our research marks a significant step forward in intraoperative 3D reconstruction, offering enhancements to surgical planning, navigation, and robotics.
Automatic registration with continuous pose updates for marker-less surgical navigation in spine surgery
Aug 05, 2023Abstract:Established surgical navigation systems for pedicle screw placement have been proven to be accurate, but still reveal limitations in registration or surgical guidance. Registration of preoperative data to the intraoperative anatomy remains a time-consuming, error-prone task that includes exposure to harmful radiation. Surgical guidance through conventional displays has well-known drawbacks, as information cannot be presented in-situ and from the surgeon's perspective. Consequently, radiation-free and more automatic registration methods with subsequent surgeon-centric navigation feedback are desirable. In this work, we present an approach that automatically solves the registration problem for lumbar spinal fusion surgery in a radiation-free manner. A deep neural network was trained to segment the lumbar spine and simultaneously predict its orientation, yielding an initial pose for preoperative models, which then is refined for each vertebra individually and updated in real-time with GPU acceleration while handling surgeon occlusions. An intuitive surgical guidance is provided thanks to the integration into an augmented reality based navigation system. The registration method was verified on a public dataset with a mean of 96\% successful registrations, a target registration error of 2.73 mm, a screw trajectory error of 1.79{\deg} and a screw entry point error of 2.43 mm. Additionally, the whole pipeline was validated in an ex-vivo surgery, yielding a 100\% screw accuracy and a registration accuracy of 1.20 mm. Our results meet clinical demands and emphasize the potential of RGB-D data for fully automatic registration approaches in combination with augmented reality guidance.
Next-generation Surgical Navigation: Multi-view Marker-less 6DoF Pose Estimation of Surgical Instruments
May 05, 2023



Abstract:State-of-the-art research of traditional computer vision is increasingly leveraged in the surgical domain. A particular focus in computer-assisted surgery is to replace marker-based tracking systems for instrument localization with pure image-based 6DoF pose estimation. However, the state of the art has not yet met the accuracy required for surgical navigation. In this context, we propose a high-fidelity marker-less optical tracking system for surgical instrument localization. We developed a multi-view camera setup consisting of static and mobile cameras and collected a large-scale RGB-D video dataset with dedicated synchronization and data fusions methods. Different state-of-the-art pose estimation methods were integrated into a deep learning pipeline and evaluated on multiple camera configurations. Furthermore, the performance impacts of different input modalities and camera positions, as well as training on purely synthetic data, were compared. The best model achieved an average position and orientation error of 1.3 mm and 1.0{\deg} for a surgical drill as well as 3.8 mm and 5.2{\deg} for a screwdriver. These results significantly outperform related methods in the literature and are close to clinical-grade accuracy, demonstrating that marker-less tracking of surgical instruments is becoming a feasible alternative to existing marker-based systems.
Automatic breach detection during spine pedicle drilling based on vibroacoustic sensing
Mar 27, 2023



Abstract:Pedicle drilling is a complex and critical spinal surgery task. Detecting breach or penetration of the surgical tool to the cortical wall during pilot-hole drilling is essential to avoid damage to vital anatomical structures adjacent to the pedicle, such as the spinal cord, blood vessels, and nerves. Currently, the guidance of pedicle drilling is done using image-guided methods that are radiation intensive and limited to the preoperative information. This work proposes a new radiation-free breach detection algorithm leveraging a non-visual sensor setup in combination with deep learning approach. Multiple vibroacoustic sensors, such as a contact microphone, a free-field microphone, a tri-axial accelerometer, a uni-axial accelerometer, and an optical tracking system were integrated into the setup. Data were collected on four cadaveric human spines, ranging from L5 to T10. An experienced spine surgeon drilled the pedicles relying on optical navigation. A new automatic labeling method based on the tracking data was introduced. Labeled data was subsequently fed to the network in mel-spectrograms, classifying the data into breach and non-breach. Different sensor types, sensor positioning, and their combinations were evaluated. The best results in breach recall for individual sensors could be achieved using contact microphones attached to the dorsal skin (85.8\%) and uni-axial accelerometers clamped to the spinous process of the drilled vertebra (81.0\%). The best-performing data fusion model combined the latter two sensors with a breach recall of 98\%. The proposed method shows the great potential of non-visual sensor fusion for avoiding screw misplacement and accidental bone breaches during pedicle drilling and could be extended to further surgical applications.
Sonification as a Reliable Alternative to Conventional Visual Surgical Navigation
Jun 30, 2022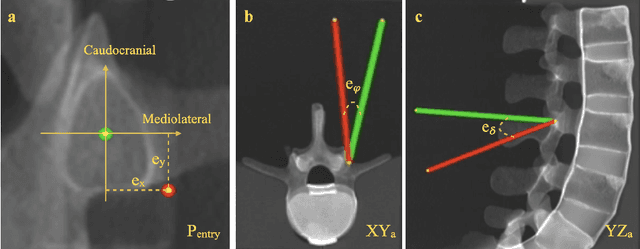
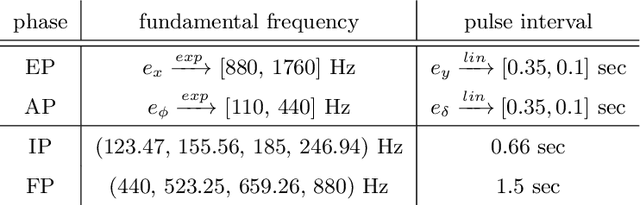
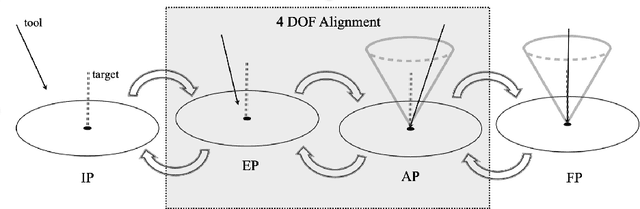
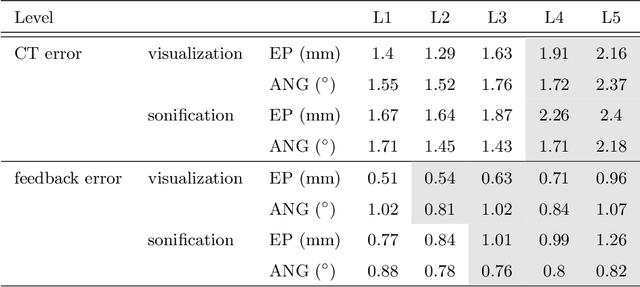
Abstract:Despite the undeniable advantages of image-guided surgical assistance systems in terms of accuracy, such systems have not yet fully met surgeons' needs or expectations regarding usability, time efficiency, and their integration into the surgical workflow. On the other hand, perceptual studies have shown that presenting independent but causally correlated information via multimodal feedback involving different sensory modalities can improve task performance. This article investigates an alternative method for computer-assisted surgical navigation, introduces a novel sonification methodology for navigated pedicle screw placement, and discusses advanced solutions based on multisensory feedback. The proposed method comprises a novel sonification solution for alignment tasks in four degrees of freedom based on frequency modulation (FM) synthesis. We compared the resulting accuracy and execution time of the proposed sonification method with visual navigation, which is currently considered the state of the art. We conducted a phantom study in which 17 surgeons executed the pedicle screw placement task in the lumbar spine, guided by either the proposed sonification-based or the traditional visual navigation method. The results demonstrated that the proposed method is as accurate as the state of the art while decreasing the surgeon's need to focus on visual navigation displays instead of the natural focus on surgical tools and targeted anatomy during task execution.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge