Jennifer Straub
Detection of Emerging Infectious Diseases in Lung CT based on Spatial Anomaly Patterns
Oct 25, 2024Abstract:Fast detection of emerging diseases is important for containing their spread and treating patients effectively. Local anomalies are relevant, but often novel diseases involve familiar disease patterns in new spatial distributions. Therefore, established local anomaly detection approaches may fail to identify them as new. Here, we present a novel approach to detect the emergence of new disease phenotypes exhibiting distinct patterns of the spatial distribution of lesions. We first identify anomalies in lung CT data, and then compare their distribution in a continually acquired new patient cohorts with historic patient population observed over a long prior period. We evaluate how accumulated evidence collected in the stream of patients is able to detect the onset of an emerging disease. In a gram-matrix based representation derived from the intermediate layers of a three-dimensional convolutional neural network, newly emerging clusters indicate emerging diseases.
Domain adaptation strategies for 3D reconstruction of the lumbar spine using real fluoroscopy data
Jan 29, 2024

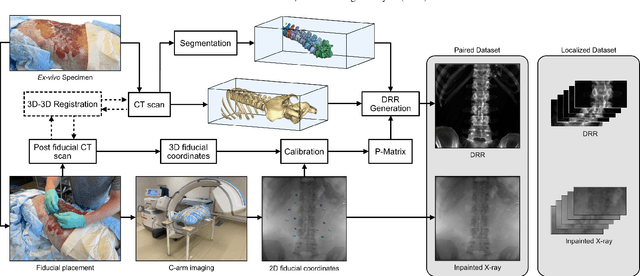

Abstract:This study tackles key obstacles in adopting surgical navigation in orthopedic surgeries, including time, cost, radiation, and workflow integration challenges. Recently, our work X23D showed an approach for generating 3D anatomical models of the spine from only a few intraoperative fluoroscopic images. This negates the need for conventional registration-based surgical navigation by creating a direct intraoperative 3D reconstruction of the anatomy. Despite these strides, the practical application of X23D has been limited by a domain gap between synthetic training data and real intraoperative images. In response, we devised a novel data collection protocol for a paired dataset consisting of synthetic and real fluoroscopic images from the same perspectives. Utilizing this dataset, we refined our deep learning model via transfer learning, effectively bridging the domain gap between synthetic and real X-ray data. A novel style transfer mechanism also allows us to convert real X-rays to mirror the synthetic domain, enabling our in-silico-trained X23D model to achieve high accuracy in real-world settings. Our results demonstrated that the refined model can rapidly generate accurate 3D reconstructions of the entire lumbar spine from as few as three intraoperative fluoroscopic shots. It achieved an 84% F1 score, matching the accuracy of our previous synthetic data-based research. Additionally, with a computational time of only 81.1 ms, our approach provides real-time capabilities essential for surgery integration. Through examining ideal imaging setups and view angle dependencies, we've further confirmed our system's practicality and dependability in clinical settings. Our research marks a significant step forward in intraoperative 3D reconstruction, offering enhancements to surgical planning, navigation, and robotics.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge